The special nanosize, nanostructure, and surface properties of nanoformulations may lead to significant changes in the behavior of the drug in vivo and in vitro, thereby achieving clinical benefits. At the same time, the safety risks caused by nanoscale effects may also increase accordingly. Therefore, in-depth research and effective control of the quality of nanoformulations are fundamental to ensure the effectiveness and safety of nanoformulations. Based on the particularities of nanoformulations such as composition, structure, physical and chemical properties, formulation processes, clinical formulation and usage methods, CD Formulation studies the quality attributes related to nanoformulations by designing, optimizing, and validating analytical and characterization methods applicable to nanoformulations.
Why is Nanoformulation Analysis Important?
Focused research and full characterization of the critical quality attributes (CQAs) of nanoformulations is not only conducive to the optimization of nanoformulation preparation process parameters and the determination of critical quality attributes, but also provides a basis for comprehensive quality control and the establishment of pharmaceutical quality standards. It is also helpful to explore the biological characteristics and mechanism of action of nanoformulations, improve the predictability of nanoformulation behavior in vivo, and provide a reference for preclinical and clinical research.
CD Formulation adheres to the specific and high-quality standards of nanoformulation, with extensive practical expertise in assessing and testing nano properties, quality control, nanoformulation analytical method development and validation, and analytical method transfer. We offer top-notch research and analytical services for nanoformulations to researchers and pharmaceutical companies.
Our Analytical Strategy for Nanoformulation
Our overall analytical strategy for quality control of nanoformulations is based on the drug evaluation risk assessment strategy. It focuses on the impact of the quality properties of nanoformulations on their safety and effectiveness. Our quality control studies based on risk assessment may include the following aspects:
- Type, composition, and structure of nanoformulations.
- The final storage form, administration route, method, therapeutic purpose, etc.
- Accuracy and applicability of nanoformulation characterization methods.
- The impact of the controllability of the nanoformulation preparation process, including intermediate control and durability of the control strategy, on the key quality attributes of the product.
- The impact of the quality performance of nanoformulation on the stability of the drug during storage and use, the in vivo release of the drug, the pharmacokinetics, in vivo distribution, biological effects, safety, and mechanism of the drug and its carrier.
Our Nanoformulation Analytical Services
Research on quality control indicators and critical quality attributes (CQAs) of nanoformulations can be used for prescription process screening and stability inspection of nanoformulations. It can provide a reference and basis for subsequent non-clinical research and even clinical research. CD Formulation, as a world-leading nanoformulation service company integrating nanoformulation development and manufacturing, characterization, and analysis evaluation, has lots of rich experience in nanoformulation development and analytical evaluation for years. Relying on our world-class advanced instruments and equipment, we can provide our client with nanoformulation analytical services including but not limited to the following items.
In addition to the above analytical services, we follow the hot spots and updated requirements of nanoformulation quality research and focus on exploring and developing unique quality analysis methods for preparations based on different nanocarriers.
Our Workflow of Nanoformulation Analytical Services
We can provide customers with professional nanoformulation analytical proposals and testing services from the following steps. To ensure that the quality of our services meets your requirements after you receive the test report, we also provide you with unique after-sales technical communication services for free.
 Fig. 1. Our workflow of nanoformulation analytical services. (CD Formulation)
Fig. 1. Our workflow of nanoformulation analytical services. (CD Formulation)
Why Choose Us for Nanoformulation Analysis?
- We hold a variety of advanced instruments and equipment, such as particle size analyzer, SEM, Zeta potentiometric meter, etc. And we have completely achieved a seamless transition from nanoformulation analytical method development to analytical method transfer.
- Our quality control and quality research team have active innovative thinking, rich nanomedicine expertise, and experimental research experience in nanoformulation analytical method development and validation. They can provide a quick response to your requirements for nanoformulation analysis immediately.
- We can provide you with various nanoformulation analytical services to match customers' different requirements, covering nanoproperty characterization, quality control and testing, analytical method development and validation, analytical method transfer, etc.
- We also provide you with free after-sales consulting and technical communication services within 15 working days from the time you receive the test report.
Published Data
Technology: Automated, Single Particle Raman Analysis
Journal: ACS Nano
IF: 17.1
Published: 2023
Results:
The authors investigated the simultaneous measurement of nanocarriers and cargo using label-free, non-destructive single particle automated Raman capture analysis (SPARTA). They first synthesized a library of model compounds that covered a range of hydrophilicities and provided unique Raman signals. These compounds are then loaded into model nanovesicles (polymersomes), which can load hydrophobic and hydrophilic cargo into the membrane or core region, respectively. The authors characterized the heterogeneity of the population by correlating signals from the membrane and cargo to each particle. This study demonstrates that this label-free analytical technology can accurately determine the cargo location and loading and release heterogeneity in nanomedicines, which provides necessary technical support for the quality control of nanoformulations. This is a confocal raman spectroscopic imaging of giant polymersomes.
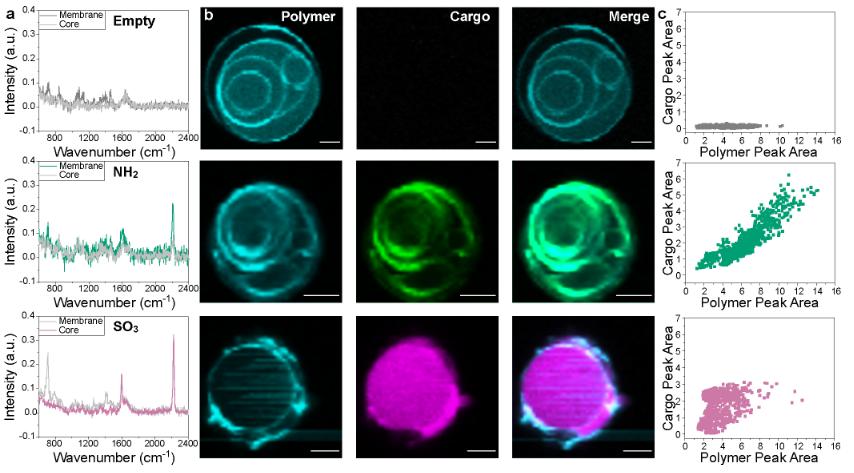 Fig.2 Confocal Raman spectroscopic imaging of giant polymersomes to determine primary loading location of different model cargoes. (Catherine Saunders, et al. 2023)
Fig.2 Confocal Raman spectroscopic imaging of giant polymersomes to determine primary loading location of different model cargoes. (Catherine Saunders, et al. 2023)
CD Formulation has been focused on the nanoformulation analytical method development and validation for many years. If you are interested in our services, please feel free to contact us by phone or email, we will reply to you within three working days.
References
- Catherine Saunders, James E. J. Foote, Jonathan P. Wojciechowski, et al. Revealing Population Heterogeneity in Vesicle-Based Nanomedicines Using Automated, Single Particle Raman Analysis. ACS Nano. 2023, 17, 11713-11728.
How It Works
STEP 2
We'll email you to provide your quote and confirm order details if applicable.
STEP 3
Execute the project with real-time communication, and deliver the final report promptly.
Related Services


 Fig. 1. Our workflow of nanoformulation analytical services. (CD Formulation)
Fig. 1. Our workflow of nanoformulation analytical services. (CD Formulation) Fig.2 Confocal Raman spectroscopic imaging of giant polymersomes to determine primary loading location of different model cargoes. (Catherine Saunders, et al. 2023)
Fig.2 Confocal Raman spectroscopic imaging of giant polymersomes to determine primary loading location of different model cargoes. (Catherine Saunders, et al. 2023)
