Drug Release Testing Service for Nanocarriers
Inquiry
Nanocarriers can potentially enhance the therapeutic efficacy of drugs by controlling the release of active drug ingredients, reducing their systemic side effects, and improving patient compliance with regimens by reducing dosage and administration frequency. By studying the release mechanism of nanocarriers, CD Formulation can provide customers with nanocarrier drug release research and testing services, assisting the development and clinical application of nanocarrier drugs.
Advantages of Nanocarriers
Nanocarriers include nanoparticles, dendrimers, and polymer or lipid carriers, such as liposomes. Nanocarriers are useful transport agents because of their small size and ability to change physical properties, such as charge and shape, to deliver active drugs into tissues. Nanocarriers serve as transport vehicles and determine the pharmacokinetics of transport and distribution. The advantages of using nanocarriers are as follows:
- Nanocarriers can prevent the degradation of active drugs, allowing for higher and more effective concentrations in target tissues, as well as reducing the severity of undesirable toxic side effects.
- Nanocarriers can be linked to specific ligands, thereby enhancing their specificity for target tissues.
- Nanocarriers are also widely used in the field of aneurysm treatment surgery.
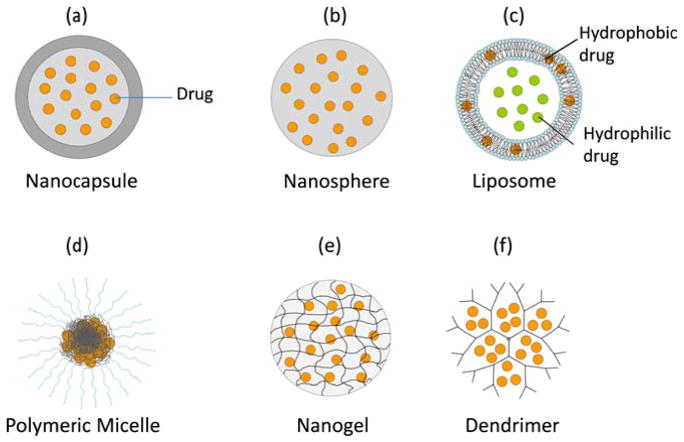 Fig.1 Different types of pharmaceutical nanocarriers for drug delivery. (Jinhyun Hannah Lee, et al. 2015)
Fig.1 Different types of pharmaceutical nanocarriers for drug delivery. (Jinhyun Hannah Lee, et al. 2015)
Research on Drug Release Mechanisms of Nanocarriers
Nanocarrier drug release is to maintain the drug concentration in the blood or target tissue at an effective level, and various mechanisms drive the drug to be released from the carriers.
Diffusion-Controlled Release
Diffusion-controlled drug release is carried out in capsule-type reservoir systems, where the drug is dissolved or dispersed in a core surrounded by a polymeric membrane, and drug diffusion is driven through the difference in its concentration across the membrane. We first dissolve the drug in a core surrounded by a polymeric membrane, and then the drug diffuses across the cell membrane.
Solvent-Controlled Release
The transport of solvent into the drug carrier affects the drug release behavior from the carrier. And the solvent-controlled release includes osmosis-controlled release and swell-controlled release.
Osmosis-controlled release occurs in a covered carrier with a semipermeable polymer membrane through which water can flow from the outside of the carrier to the drug-loaded core. That is to say, it flows from low drug concentration to high drug concentration, maintaining a constant concentration gradient across the membrane, thereby generating a zero-order release curve.
Swelling-controlled release is when a glassy hydrophilic polymer system is placed in an aqueous solution containing body fluids, and the absorption of water causes the polymer particles to swell, followed by drug release. Swelling-controlled systems may achieve zero-order drug release, with the rate of drug release determined by the diffusion rate of water and the chain relaxation rate of the polymer.
Degradation-Controlled Release
Degradation-controlled release includes biodegradable polymers, (such as polyesters, polyamides, polyamino acids) and polysaccharides release the drug through hydrolysis and/or enzymatic degradation of ester, amide, and hydrazone linkages in their backbones. The drug release kinetics are determined by the degradation rate of polymers, which is up to their molecular weight, end groups, monomer composition, and crystallinity.
Stimuli-Controlled Release
The drug release from responsive nanocarriers is regulated by internal or external stimuli like temperature, pH, ionic strength, sound, and electric or magnetic. The application of these carriers for delivering drugs to specific targets has been extensively researched and investigated due to their capability to concentrate stimuli.
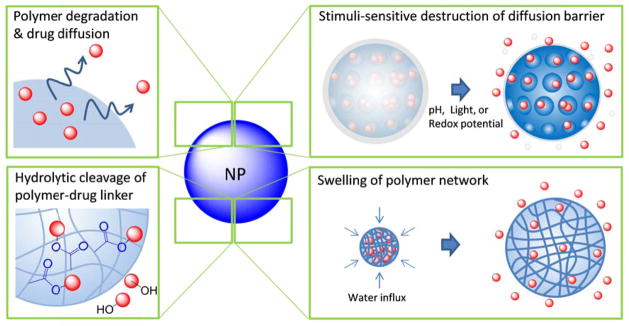 Fig.2 Drug release mechanisms utilized in nanocarriers. (Jinhyun Hannah Lee, et al. 2015)
Fig.2 Drug release mechanisms utilized in nanocarriers. (Jinhyun Hannah Lee, et al. 2015)
Our Methods for Nanocarrier Drug Release Testing
We can offer drug release from different nanocarrier testing services relying on our rich experience in exploring and researching on mechanism of drug release from nanocarriers by the following testing methods.
 Fig.3 Our different nanocarrier drug release testing methods. (CD Formulation)
Fig.3 Our different nanocarrier drug release testing methods. (CD Formulation)
Why Choose Us to Test Drug Release from Nanocarriers?
- We have been studying drug release mechanisms from nanocarriers for many years and use this to guide the formulation development and clinical research of nanocarrier-based formulations.
- We have a professional nanoformulation development team with rich practical experience in drug release testing in nanocarriers, which provides us with the necessary professional technical support to undertake this type of testing services quickly.
- We also studied the release pharmacokinetic mechanisms of different nanocarriers, which provided necessary technical support for the modification of nanocarriers or testing of drug release kinetics in vitro during nanoformulation development.
Published Data
Technology: Laser desorption/ionization MS imaging strategy for evaluation of in vivo and in situ drug release of nanocarriers
Journal: Science Advances
IF: 13.6
Published: 2018
Results:
The authors developed a novel label-free laser desorption/ionization mass spectrometry (MS) imaging strategy to visualize and quantify in situ drug release in tissues by monitoring the intrinsic MS signal intensity ratio of drug-loaded drugs on nanocarriers. By studying the doxorubicin (DOX)/polyethylene glycol-MoS nanosheet drug delivery system in tumor mouse models. This new strategy opens new avenues for studying in vivo and in situ drug release from drug carrier systems such as carbon nanotubes and black phosphorus nanosheets and for evaluating drug release from nanocarriers at the suborgan level. Here is the evaluation of in vivo and in situ drug release of MoS2 nanocarriers.
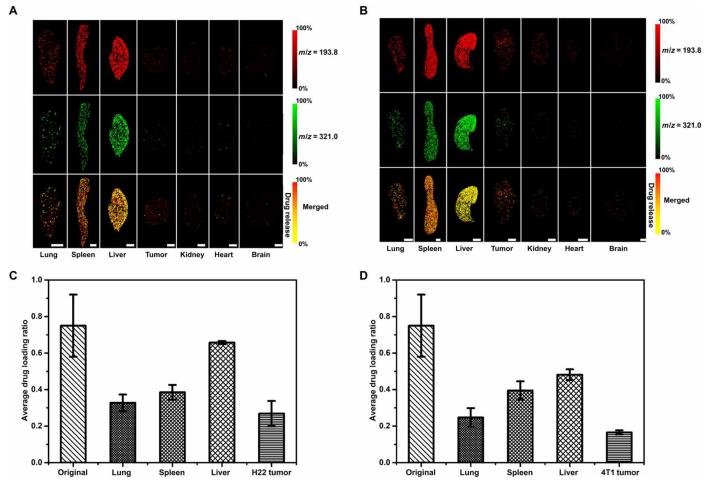 Fig.4 Biodistribution and drug release studies of DOX/PEG-MoS2 revealed by LDI MSI. (Jinjuan Xue, et al. 2018)
Fig.4 Biodistribution and drug release studies of DOX/PEG-MoS2 revealed by LDI MSI. (Jinjuan Xue, et al. 2018)
As a leader in the development and clinical transformation of nanoformulation, CD Formulation has deeply explored and studied various possible releases of nanocarriers and in vitro release pharmacokinetic mechanisms and can provide drug release form nanocarrier testing and in vitro release pharmacokinetics for global nanoformulation researchers. If you are interested in our services, please contact us via phone or email.
References
- Jinhyun Hannah Lee, Yoon Yeo. Controlled Drug Release from Pharmaceutical Nanocarriers. Chem Eng Sci. 2015,125: 75-84.
- Jinjuan Xue, Huihui Liu, Suming Chen. et al. Mass spectrometry imaging of the in situ drug release from nanocarriers. Science Advances. 2018,4(10): eaat9039.
How It Works
STEP 2
We'll email you to provide your quote and confirm order details if applicable.
STEP 3
Execute the project with real-time communication, and deliver the final report promptly.
Related Services


 Fig.1 Different types of pharmaceutical nanocarriers for drug delivery. (Jinhyun Hannah Lee, et al. 2015)
Fig.1 Different types of pharmaceutical nanocarriers for drug delivery. (Jinhyun Hannah Lee, et al. 2015) Fig.2 Drug release mechanisms utilized in nanocarriers. (Jinhyun Hannah Lee, et al. 2015)
Fig.2 Drug release mechanisms utilized in nanocarriers. (Jinhyun Hannah Lee, et al. 2015) Fig.3 Our different nanocarrier drug release testing methods. (CD Formulation)
Fig.3 Our different nanocarrier drug release testing methods. (CD Formulation) Fig.4 Biodistribution and drug release studies of DOX/PEG-MoS2 revealed by LDI MSI. (Jinjuan Xue, et al. 2018)
Fig.4 Biodistribution and drug release studies of DOX/PEG-MoS2 revealed by LDI MSI. (Jinjuan Xue, et al. 2018)
