Nanoformulation Critical Micelle Concentration Testing Service
Inquiry
Critical micelle concentration (CMC) is a fundamental parameter characterizing micellization with a closed association mechanism. CMC is the polymeric concentration above which micelles form. CD Formulation can determine critical micelle concentration (CMC) of polymeric micelles through measuring the interfacial tension, or surface tension, at different surfactant concentrations.
What is Critical Micelle Concentration (CMC)?
Critical micelle concentration (CMC) is the concentration of surfactant at which micelles first form in solution. The CMC value is related to the structure of the molecule. The addition of surfactant reduces the interfacial energy in the solution and eliminates hydrophobic groups on the surface of the surfactant in contact with water. Instead, the free energy of the system decreases. As the concentration of surfactant in a solution increases, the molecules of the surfactant begin to aggregate with other molecules and form micelles. The formation of micelles sharply reduces the free energy of the solution by weakening the interaction between the hydrophobic groups and the surfactant, and the surfactant concentration reaches the CMC. Increasing surfactant dosage above the CMC value causes more micelles to form but hardly decreases the free energy of the solution. Below the CMC value, excess surfactant in the solution remains in monomeric form, above this value all additional surfactant creates micelles.
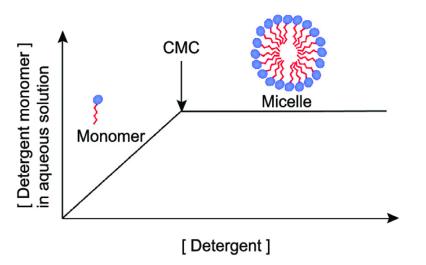 Fig.1 Schematic definition of the critical micelle concentration. (Ehsan Kamaei, et al. 2019)
Fig.1 Schematic definition of the critical micelle concentration. (Ehsan Kamaei, et al. 2019)
Our Critical Micelle Concentration Testing Techniques
Critical micelle concentration (CMC) is the concentration of surfactant above which micelles form and all other surfactants added to the system will form micelles. CD Formulation determines the surfactant solution by measuring the interfacial tension, or surface tension, at different surfactant concentrations utilizing tension measurement, conductimetry, density measurement, fluorescence spectrum measurement and high-resolution ultrasonic spectroscopy.
Tensiometric Measurements
The critical micelle-forming concentration can be determined graphically with the help of a logarithmic plot: the value of the interfacial or surface tension is entered on the y-axis and the surfactant concentration on the logarithmic x-axis.
Conductivity Measurements
When the surfactant in the solution is present in the form of micelles, the increase in conductivity per unit concentration is higher and the CMC can be calculated from the breakpoints of the raw data.
Density Measurements
Density changes with changes in the aggregation state of the surfactant. The increase in volume of a surfactant as a unit mass is less than the increase in volume of a micelle. For all surfactants analyzed, the CMC showed a stepwise deflection as density increased with concentration.
Fluorescence Spectroscopy Measurements
As the microenvironment around the pyrene (used as a fluorescent probe) changes, the hydrophobicity of the surfactant concentration changes as a result of the interaction between the pyrene and the surfactant micelles.
HR-US Measurements
HR-US measures how the properties of ultrasonic waves change after passing through the material. This spectroscopic technique uses high-intensity ultrasound at low frequencies (20−100 kHz) to study the structural properties of materials as a function of concentration or temperature in a fast, non-destructive and reliable way.
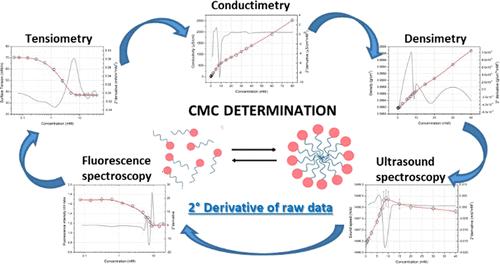 Fig.2 CMC determination methods. (Diego Romano Perinelli, et al. 2020)
Fig.2 CMC determination methods. (Diego Romano Perinelli, et al. 2020)
Our Critical Micelle Concentration Testing Process
Our team is committed to research the CMC determination for years and can undertake your project about CMC testing requirements, and there are just four steps for our CMC determination process.
 Fig.3 Our workflow of CMC testing. (CD Formulation)
Fig.3 Our workflow of CMC testing. (CD Formulation)
Why Choose Us to Test Nanoformulation Critical Micelle Concentration?
- We can test critical micelle concentrations (CMC) of polymeric micelles utilizing different techniques such as tension measurement, conductimetry, density measurement, fluorescence spectrum measurement and high-resolution ultrasonic spectroscopy.
- We have a technical team with rich experience in nanoformulation development and nanoproperty characterization. And they are fully qualified to handle your CMC measurement project and can efficiently complete and provide you with our professional test reports.
- Our CMC testing process is simple and easy to operate and can provide you with CMC testing results quickly and accurately.
Published Data
Technology: Two strategies for the prediction of CMC
Journal: Journal of Colloid and Interface Science
IF: 9.9
Published: 2022
Results:
The authors present a newly developed method using first-principles interfacial tension calculations based on COSMO-RS theory for predicting the critical gel for a set of nonionic, cationic, anionic and zwitterionic surfactants in water. beam concentration. Their approach consists of a combination of two predictive strategies for simulating two different phenomena, including the removal of hydrophobic tails of surfactants in contact with water. Both strategies are based on regular micelle formation and thermodynamic phase separation of surfactants in water, and both require consideration of a wide range of polarities in the hydrophilic headgroups. This method can accurately predict the critical micelle concentration and is applicable to various surfactant types.
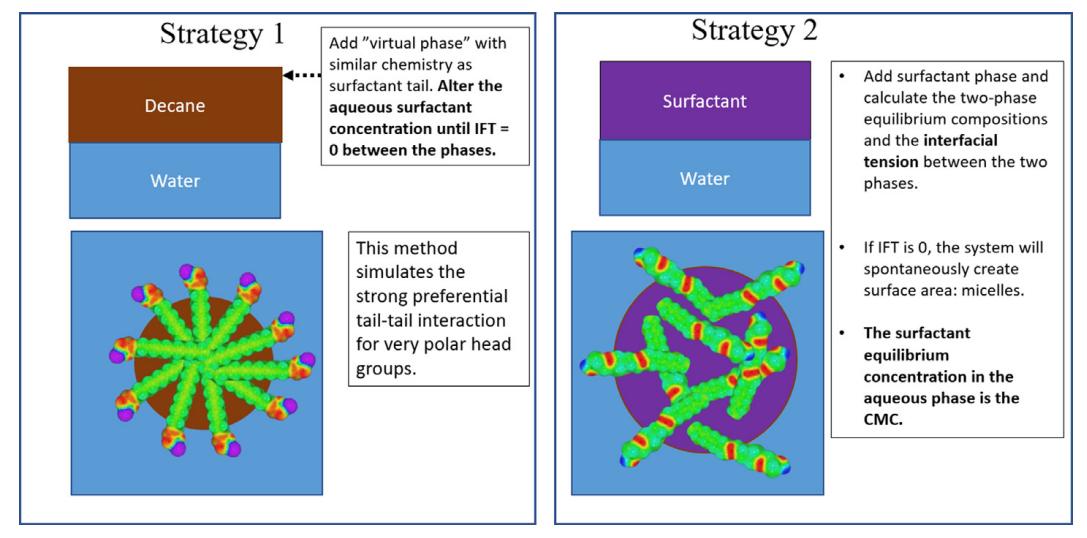 Fig.4 Schematic representation of Strategies 1 and 2 for the prediction of CMC. (M Turchi, et al. 2022)
Fig.4 Schematic representation of Strategies 1 and 2 for the prediction of CMC. (M Turchi, et al. 2022)
CD Formulation has conducted in-depth research on critical micelle concentration testing technology. It can select the appropriate CMC testing technology according to the different testing requirements of customers and calculate the CMC value quickly and accurately through the second-order derivative method. If you have any questions about CMC determination, please kindly reach out to us for detailed communication.
References
- Ehsan Kamaei, Abbas Khaksar Manshada, Seyed Reza Shadizadeh, et al. Effect of the wettability alteration on the cementation factor of carbonate rocks using Henna extract. Materialia. 2019, 8: 100440.
- Diego Romano Perinelli, Marco Cespi, Nicola Lorusso, et al. Surfactant Self-Assembling and Critical Micelle Concentration: One Approach Fits All? Langmuir. 2020, 36, 5745-5753.
- M Turchi, A P Karcz, M P Andersson. First-principles prediction of critical micellar concentrations for ionic and nonionic surfactants. Journal of Colloid and Interface Science. 2022,606: 618-627.
How It Works
STEP 2
We'll email you to provide your quote and confirm order details if applicable.
STEP 3
Execute the project with real-time communication, and deliver the final report promptly.
Related Services


 Fig.1 Schematic definition of the critical micelle concentration. (Ehsan Kamaei, et al. 2019)
Fig.1 Schematic definition of the critical micelle concentration. (Ehsan Kamaei, et al. 2019) Fig.2 CMC determination methods. (Diego Romano Perinelli, et al. 2020)
Fig.2 CMC determination methods. (Diego Romano Perinelli, et al. 2020) Fig.3 Our workflow of CMC testing. (CD Formulation)
Fig.3 Our workflow of CMC testing. (CD Formulation) Fig.4 Schematic representation of Strategies 1 and 2 for the prediction of CMC. (M Turchi, et al. 2022)
Fig.4 Schematic representation of Strategies 1 and 2 for the prediction of CMC. (M Turchi, et al. 2022)
