In Vitro Release Determination of Nanoformulations
Inquiry
In order to ensure the safe use of nanodrug delivery systems, it is very necessary to measure the in vitro release of drugs in nano-drug delivery systems and observe the in vitro release behavior of drugs in nanodrug delivery systems. CD Formulation The main methods for measuring the in vitro release of nanomedicines include dialysis, centrifugation, flow cell method, gel method, etc.
Why Conduct In Vitro Release Determination of Nanoformulations?
The in vitro release of nanomedicine refers to the speed and degree of drug release from sustained-release formulations, controlled-release formulations, enteric-coated formulations and transdermal patches under specified conditions. It is an important indicator for evaluating the quality of nanomedicine delivery systems. By measuring the in vitro release of the nano-delivery system and establishing a mathematical model related to in vivo and in vitro release, we can study its in vivo and in vitro correlations to predict its in vivo behavior and control its quality.
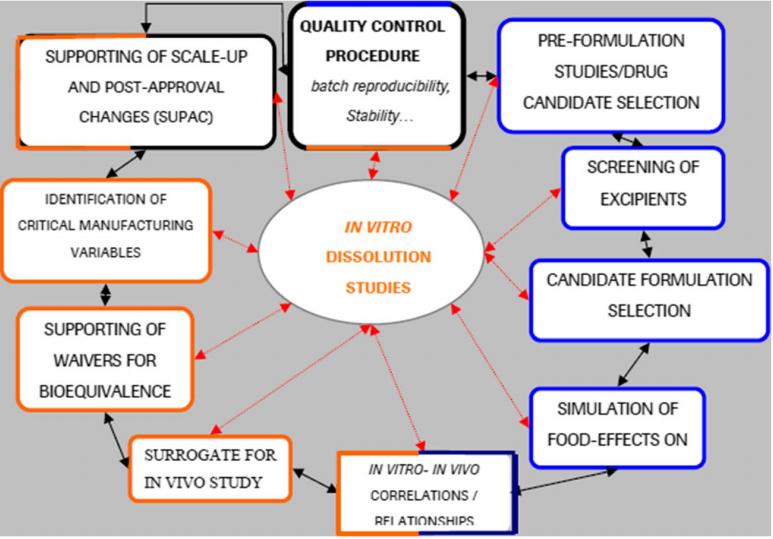 Fig.1 The central roles of in vitro dissolution testing. (Ravi Sheshala, et al. 2019)
Fig.1 The central roles of in vitro dissolution testing. (Ravi Sheshala, et al. 2019)
Therefore, while examining the drug release in vitro, relevant in vivo pharmacokinetic tests should be established, which is of great significance to product quality control, production process optimization, and bioequivalence research.
Our In Vitro Release Determination Service of Nanoformulations
Our team can quickly respond to you in vitro release test requirements of nanoformulations, and we can provide our analytical proposal to meet your analytical needs.
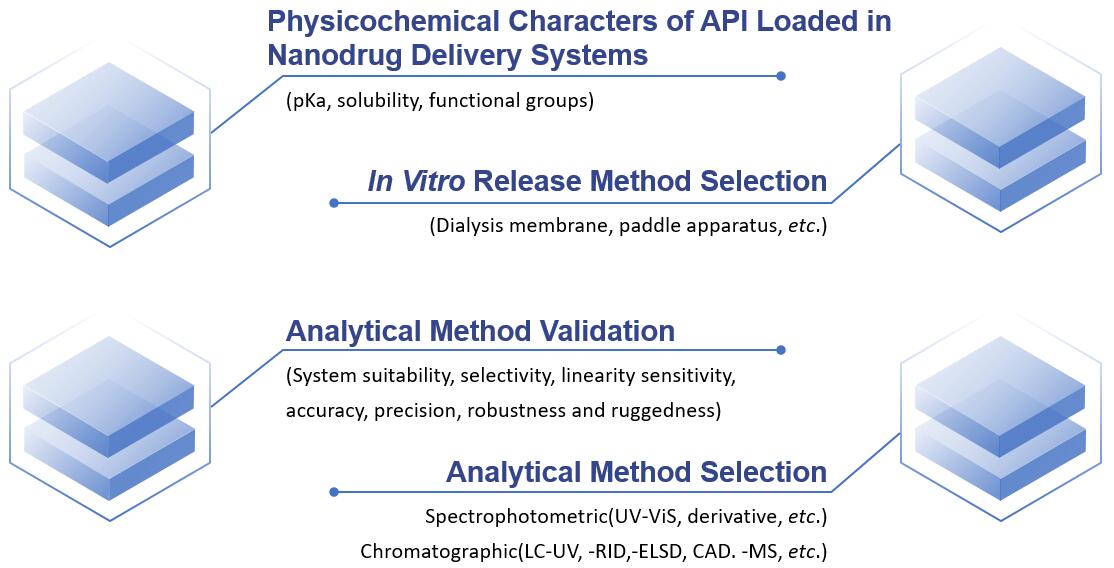 Fig.2 Our workflow of in vitro release determination of nanoformulations. (CD Formulation)
Fig.2 Our workflow of in vitro release determination of nanoformulations. (CD Formulation)
Our In Vitro Drug Release of Nanoformulations Methods
In vitro release testing is a key physical parameter for determining nanoformulation quality, monitoring formulation design, and batch-to-batch variation. CD Formulation has a strong focus on developing appropriate methods to evaluate the in vitro release of nanoformulations, such as sample and separate, membrane-free dissolution, continuous flow, dialysis assay, etc.
Sample and Separate
In this method, a nanoparticle dosage form is introduced into a release medium maintained at a constant temperature, and drug release is then assessed by sampling the release medium (filtrate or supernatant) or nanoparticles.
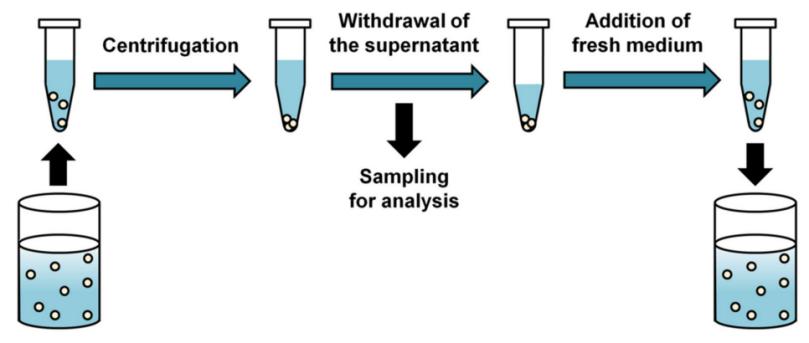 Fig.3 Basic principle and process of sample and separate method for in vitro drug release testing procedure. (Yejin Kim, et al. 2021)
Fig.3 Basic principle and process of sample and separate method for in vitro drug release testing procedure. (Yejin Kim, et al. 2021)
Membrane-Free Dissolution Method
In the membrane-free approach, nanocarriers are introduced directly into the release medium that is maintained at a constant temperature. We assess drug release by physically separating the nanocarriers from the release medium at predetermined time intervals via sample separation techniques (filtration/centrifugation/centrifugal ultrafiltration) and subsequently quantify the amount of drug in the filtrate/supernatant using appropriate analytical techniques.
Continuous Flow
In this method, we monitor drug release from nanoparticle dosage forms using the USP IV device or its modifications. Buffer or medium is continuously circulated through a column containing a fixed dosage form, whereby drug release occurs and is monitored by periodically collecting the eluate.
Dialysis Method
Dialysis assay (DM) is the most versatile and popular of all methods for assessing drug release from nanodosage forms. In this method, physical separation of dosage forms is achieved through the use of dialysis membranes.
Electrochemical Methods
Electrochemical methods offer the possibility of rapid in situ measurements while avoiding interferences caused by the presence of undissolved dosage forms in the release medium.
Microdialysis
Microdialysis is based on the passive diffusion of a drug through a concentration gradient across a semipermeable membrane and the use of analytical methods such as HPLC to measure drug release under real-time conditions.
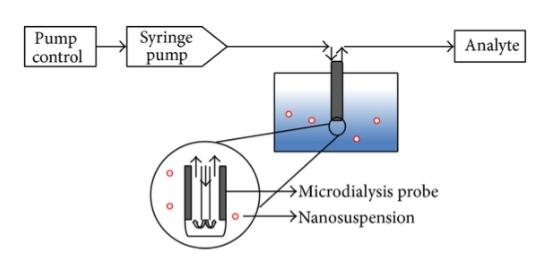 Fig.4 Microdialysis set-up. (Susan D’ Souza. 2014)
Fig.4 Microdialysis set-up. (Susan D’ Souza. 2014)
In addition to the above methods, we also explored non-electrochemical methods such as calorimetry, turbidimetry, and laser diffraction as evaluation methods for in vitro release.
Why Choose Us to Determine In Vitro Release of Nanoformulations?
- We have explored many in vitro release methods, such as sample and separate, membrane-free dissolution, continuous flow, dialysis assay, etc.
- Our sample circulation cycle is relatively fast, and we can quick response to your detailed requirements for in vitro release determination of nanoformulations.
- We have rich experience in vitro drug release testing of nanoformulations and can quickly provide you with our professional services your project, with comprehensive analytical proposal design and in vitro drug release testing method development and validation.
Published Data
Technology: Adaptive perfusion method
Journal: Journal of Controlled Release
IF: 10.8
Published: 2021
Results:
The authors developed an adaptive perfusion method based on the principle of tangential flow filtration (TFF), a pressure-driven separation method for studying the separation of pharmaceutical products containing particulates such as emulsions, suspensions, liposomes, The rate and extent of drug release from a drug). The adaptive perfusion method enables particle size separation and simultaneous analysis of released and remaining drug. Adaptive perfusion methods can be used to measure the rate and extent of drug release from drug solutions, drug-loaded micelles and nanoemulsions by adjusting the filter's molecular weight cutoff, feed flow rate, or backpressure. The authors also introduce that the IVRT method is not limited by rate-limiting factors (such as diffusion through dialysis membranes) and has the potential to be further extended to examine the impact of the manufacturing process on drug distribution and release characteristics of other challenging complex pharmaceutical products. The adaptive perfusion method provides differentiated drug release profiles for drugs in solutions, micelles, and small, medium, and large spherical nanoemulsions. The authors found that drug release profiles obtained using the adaptive perfusion method were significantly faster (e.g., minutes instead of hours) and higher (e.g., >60%) than those obtained using the dialysis method.
CD Formulation has a strong R&D team with rich experience in vitro release determination of nanoformulation and can provide you with our professional in vitro release determination services to meet your nanoformulation development and manufacturing requirements. If you are interested in our in vitro release testing services, please feel free contact us.
References
- Ravi Sheshala, Nor Khaizan Anuar, Nor Hayati Abu Samah, et al. In Vitro Drug Dissolution/Permeation Testing of Nanocarriers for Skin Application: a Comprehensive Review. AAPS PharmSciTech. 2019,20: 164.
- Yejin Kim, Eun Ji Park, Tae Wan Kim, et al. Recent Progress in vitro Drug Release Testing Methods of Biopolymeric Particulate System. Pharmaceutics. 2021,13,1313.
- Susan D’ Souza. A Review of In Vitro Drug Release Test Methods for Nano-Sized Dosage Forms. Advances in Pharmaceutics. 2014, doi.org/10.1155/2014/304757.
- Deval Patel, Ying Zhang, Yixuan Dong, et al. Adaptive perfusion: An in vitro release test (IVRT) for complex drug products. Journal of Controlled Release. 2021, 333: 65-75.
How It Works
STEP 2
We'll email you to provide your quote and confirm order details if applicable.
STEP 3
Execute the project with real-time communication, and deliver the final report promptly.
Related Services

 Fig.1 The central roles of in vitro dissolution testing. (Ravi Sheshala, et al. 2019)
Fig.1 The central roles of in vitro dissolution testing. (Ravi Sheshala, et al. 2019) Fig.2 Our workflow of in vitro release determination of nanoformulations. (CD Formulation)
Fig.2 Our workflow of in vitro release determination of nanoformulations. (CD Formulation) Fig.3 Basic principle and process of sample and separate method for in vitro drug release testing procedure. (Yejin Kim, et al. 2021)
Fig.3 Basic principle and process of sample and separate method for in vitro drug release testing procedure. (Yejin Kim, et al. 2021) Fig.4 Microdialysis set-up. (Susan D’ Souza. 2014)
Fig.4 Microdialysis set-up. (Susan D’ Souza. 2014)
