Endotoxin and Sterility Testing Service of Injectable Nanoformulations
Inquiry
Endotoxins are derived from the cell walls of Gram-negative bacteria and are composed of molecules that include both lipid and complex sugar components. Endotoxins are lethal to humans because they trigger the innate immune system, causing disease. Lipopolysaccharide inflammation is characterized by fever, chills, and septic shock. Sufficiently high concentrations of endotoxin in sterile injectable nanoformulations can cause death through lipopolysaccharide inflammation. CD Formulation can provide bacterial endotoxin testing and sterility testing services to researchers and related industries according to the quality and safety requirements of injectable nanoformulations.
Why Conduct Endotoxin and Sterility Testing of Injectable Nanoformulations?
Endotoxins are molecules produced by Gram-negative bacteria that can contaminate medical products with deadly consequences. They are excreted by bacteria such as Escherichia coli (E. coli) and Salmonella during cell growth, cell division, and especially cell death. They are a type of pyrogen and a substance that causes fever.
Endotoxins may enter medical products from a variety of sources, including raw materials, excipients, bacterial production systems, equipment and packaging during manufacturing, and injectable nanoformulations. Bacteria can be used to produce pharmaceutical substances, and endotoxins may inadvertently co-purify with desired molecules.
Requirements for sterility and endotoxins vary based on nanoformulation formulation and route of administration. Many nanoformulations may not be sterilizable through conventional terminal sterilization procedures due to their complex composition and structure, so sterility testing is very important for nanoformulations. Therefore, it is necessary for us to evaluate endotoxin levels and sterility in injected nanoformulations.
Our Endotoxin Testing of Injectable Nanoformulations
As we evaluate the immunotoxicity and safety of nanoformulations, the detection of sterility and bacterial endotoxins is very important. We usually use limulus amebocyte lysate (LAL) method to measure endotoxin. The LAL method has three forms, such as color development, turbidity and gel detection. In some cases, nanoparticles may interfere with LAL determination, resulting in inaccurate or poorly reproducible results. Through years of experimental research, we have found that common interferences include colored nanoformulations that interfere with fluorescence measurements, nanosuspensions that interfere with turbidity measurements, and nanoparticles filtered with cellulose filters that produce false positives. When a certain LAL method is interfered with the measurement, we will consider using another measurement form.
Acceptable endotoxin levels depend on the type of injectable nanoformulation, disease indication, and dose administered. And our endotoxin testing process is as follows.
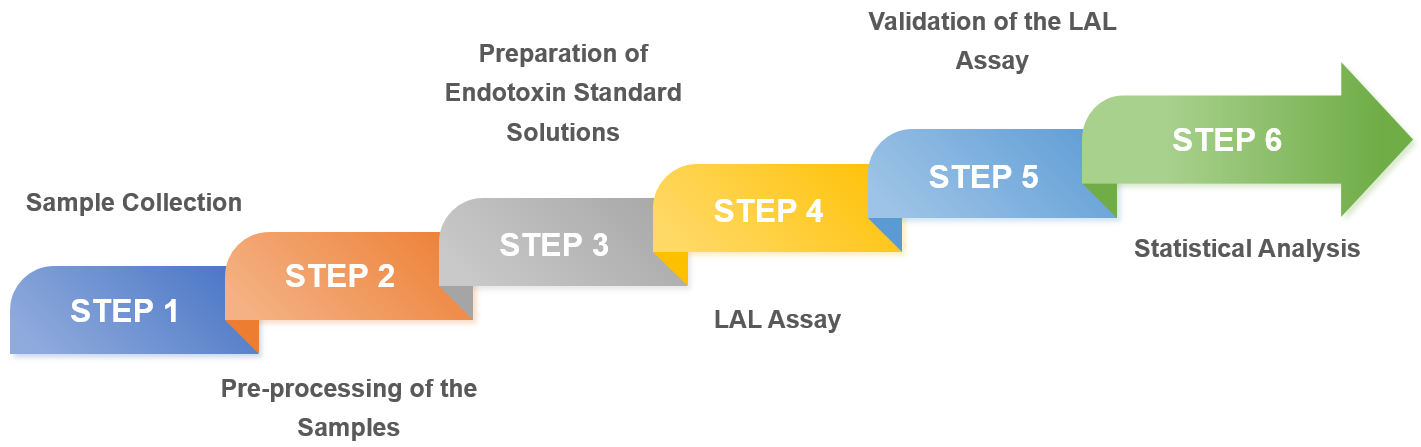 Fig.1 Our endotoxin testing services process. (CD Formulation)
Fig.1 Our endotoxin testing services process. (CD Formulation)
Our Endotoxin Testing Methods of Injectable Nanoformulations
Our team has explored and researched variety of endotoxin detection methods to meet our customers’ different texting requirements for injectable nanoformulation during their formulation development, production and quality control.
Conventional Method of Endotoxin Detection
Rabbit pyrogen test (RPT): The detection principle of RPT is to inject the drug into the rabbit and observe the temperature rise or fever of the animal. The method can detect endotoxin concentrations up to 0.5 EU/ml.
Limulus amebocyte lysate (LAL) test: The LAL test uses a blood extract from the horseshoe crab (Limulus limulus) and is often called an indirect animal test. The method can be divided into three basic techniques: gel clotting, turbidimetry and chromogenic techniques. It can detect only 0.03 EU/ml.
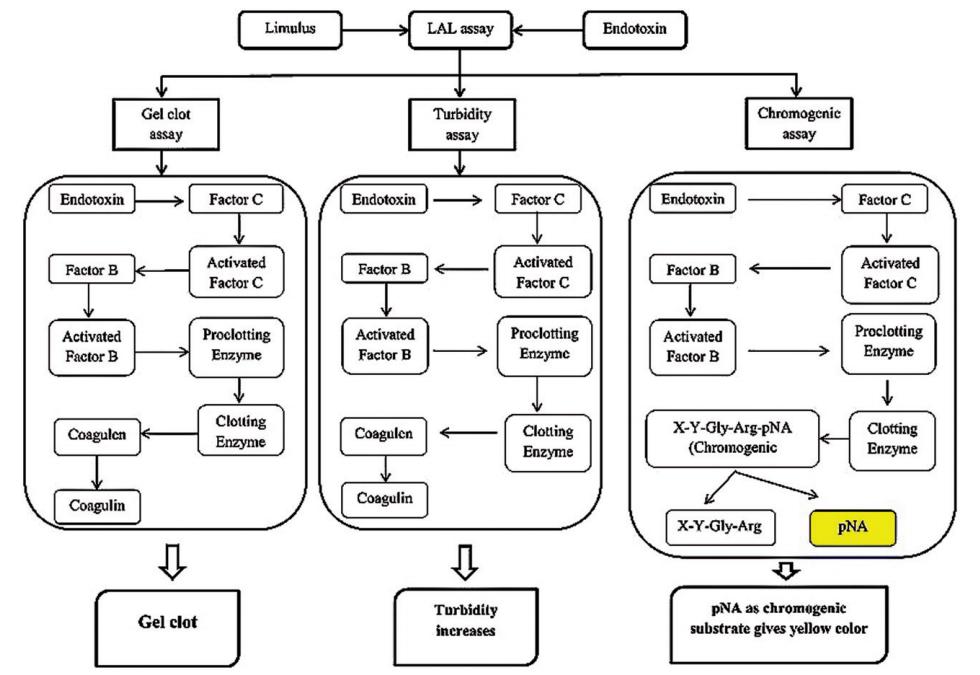 Fig.2 Types of LAL-based endotoxin detection methods. (Elvina Clarie Dullah, et al. 2017)
Fig.2 Types of LAL-based endotoxin detection methods. (Elvina Clarie Dullah, et al. 2017)
Monocyte activation test (MAT): The principle of MAT is to expose human blood directly to the surface of the test material and then quantify the amount of the pro-inflammatory cytokine IL-1b. This method can detect endotoxin concentrations as low as 10 pg/ml, and even lower using cryopreserved blood.
New Biosensors for Endotoxin Detection
Electrochemical biosensors: Electrochemical impedance spectroscopy (EIS) is a detection method based on principal component analysis (PCA), cluster analysis (CA), and multivariable discriminant analysis (MDA). The biosensing electrode surface was modified with a highly selective endotoxin neutralizing protein (ENP). The resulting biosensor is able to distinguish lipopolysaccharide from mixtures of proteins, nucleic acids and phospholipids and detect lipopolysaccharides below thresholds set by the pharmaceutical industry.
Optical biosensors: Optical biosensor utilizes mutant firefly luciferase for endotoxin detection. The optical biosensing principle is a combination of LAL reaction and bioluminescence using mutant luciferase. This method can detect 0.0005 EU/ml endotoxin in 15 minutes.
Mass-based biosensors: Mass-based biosensors use an electromagnetic piezoacoustic sensing (EMPAS) platform to detect endotoxins in blood samples.
Our Sterility Testing Methods
Sterility testing is to inoculate the nanoformulation test sample or its extract into the culture medium to check whether there is microbial contamination in the test sample. We perform sterility testing of nanoformulations via direct inoculation or membrane filtration methods, which can be performed in an isolator or clean room environment.
Why Choose Us to Test Endotoxin and Sterility of Injectable Nanoformulations?
- We can provide endotoxin and sterility testing services of injectable nanoformulation in order to support customers’ injectable nanoformulation development and quality control needs.
- Our core team have rich experience in exploring and research special endotoxin detection methods, especially new biosensors based on advanced design concept and professional techniques.
- We also provide our customers with endotoxin testing method development and validation services when it is necessary.
Published Data
Technology: UV-Vis absorption
Journal: WIREs Nanomedicine and Nanobiotechnology
IF: 8.6
Published: 2021
Results:
The author studied and summarized four commonly used methods for endotoxin detection of nanomaterials, namely rabbit pyrogen test (RPT), Limulus reagent amoeba cell lysate (LAL), monocyte activation test (MAT) and recombinant factor C (rFC) test, and alternative endotoxin detection methods have been explored, such as high-performance liquid chromatography and mass spectrometry (HPLC-MS), Macrophage activation test, and TLR4 reporter assay. At the same time, the authors evaluated the interference of nanomaterials on endotoxin contamination using MTT, LDH and ELISA, and explored strategies to avoid endotoxin contamination in nanomaterials. This research is of great significance in the clinical translation application of nanomaterials.
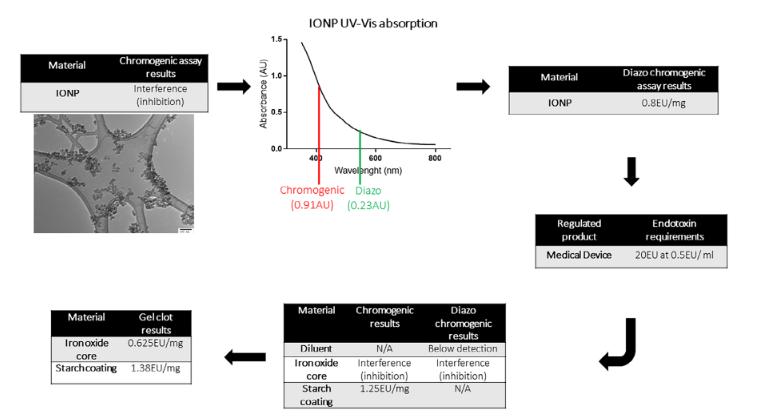 Fig.3 Endotoxin assessment of a leading IONP. (Gary Hannon, et al. 2021)
Fig.3 Endotoxin assessment of a leading IONP. (Gary Hannon, et al. 2021)
CD Formulation has advanced analytical design concept and a strong team with rich experience to provide endotoxin and sterility testing services during your injectable nanoformulation development and manufacturing. If you are interested in our endotoxin and sterility testing services, please kindly feel free to contact us for your detailed information.
References
- Elvina Clarie Dullah, Clarence M. Ongkudon.Current trends in endotoxin detection and analysis of endotoxin-protein interactions. Critical Reviews in Biotechnology. 2017, 37(2): 251-261.
- Gary Hannon, Adriele Prina-Mello. Endotoxin contamination of engineered nanomaterials: Overcoming the hurdles associated with endotoxin testing. WIREs Nanomedicine and Nanobiotechnology. 2021,13:e1738.
How It Works
STEP 2
We'll email you to provide your quote and confirm order details if applicable.
STEP 3
Execute the project with real-time communication, and deliver the final report promptly.
Related Services


 Fig.1 Our endotoxin testing services process. (CD Formulation)
Fig.1 Our endotoxin testing services process. (CD Formulation) Fig.2 Types of LAL-based endotoxin detection methods. (Elvina Clarie Dullah, et al. 2017)
Fig.2 Types of LAL-based endotoxin detection methods. (Elvina Clarie Dullah, et al. 2017) Fig.3 Endotoxin assessment of a leading IONP. (Gary Hannon, et al. 2021)
Fig.3 Endotoxin assessment of a leading IONP. (Gary Hannon, et al. 2021)
