Nanoformulation Mean Particle Size Distribution and Testing Service
Inquiry
Particle size distribution is the proportion of particles of various sizes in nanocarrier particles or nanopreparations to the total number of particles. The particle size of nanomedicines can directly affect drug solubility and dissolution rate, thereby affecting clinical efficacy. CD Formulation can provide our clients with mean particle size distribution and testing services for APIs, nanocarriers, excipients, and nanoformulations with the help of our advanced equipment.
Why Control Nanoformulation Particle Size?
Many of the unique properties of nanocarriers or nanoformulations are determined by parameters such as the average size and size distribution of the particles. The particle size distribution of a nanoformulation relates to the extent to which the quality of the nanoformulation is stable or variable. The particle size of nanopreparations not only affects the drug loading and release behavior of active ingredients, but is also closely related to pharmacokinetics, biodistribution, clearance pathways, etc., and may even be related to the delivery mechanism of nanoformulations.
The particle size and distribution of nanopreparations have an important impact on their quality and efficacy and are important quality control indicators of nanoformulations. Therefore, precise control of particle size and distribution is necessary to ensure the quality and stability of nanoformulations. During the development and manufacturing of nanoformulations, we attach great importance to controlling the average particle size and distribution of API, nanocarriers, excipients, and nanoformulations (such as liposomes, microspheres, nanoparticles, etc.) to ensure that they are suitable for various drug delivery systems.
Our Mean Particle Size Distribution and Testing Techniques
With many years of experience in the development and research of nanoformulation, CD Formulation usually selects appropriate measurement methods to study the particle size and distribution of nanoformulations and conducts complete methodological verification and optimization. We have long been engaged in exploring and studying the most effective particle size distribution determination technology for nanocarriers and nanoformulations. We can provide customers with the most economical, affordable, and effective particle size determination methods to support customers' nanoformulation development and quality control.
Dynamic Light Scattering (DLS)
Usually, we use dynamic light scattering (DLS) to measure particle size and distribution and use certified reference material (CRM) for verification. The measurement result is hydrodynamic particle size (Rh). The polydispersity index (PDI) is used to express particle size distribution.
Transmission Electron Microscopy (TEM)
Observation with the TEM is the most commonly used and intuitive method to detect the size and distribution of nanoparticles. What we measure with this method is the particle size rather than the grain size, and the nanoparticle sizes range from 0.1 nm to 1 μm.
X-Ray Small-Angle Scattering
We use this method to determine the particle size distribution of ultrafine particles with particle sizes ranging from 1 to 300 nm, including inorganic, organic sol and biological macromolecule particle sizes.
Laser method
With the help of a laser scattering particle size analyzer, we can measure nanoparticles in the range of 2 nm - 10 μm.
CD Formulation, as the world's leading pharmaceutical development and analytical services company, can offer a comprehensive and timely mean particle size distribution testing proposal and helps you quickly test the particle size distribution of the APIs, nanocarriers, and nanoformulations. In addition, we also explored microscopic imaging techniques, such as scanning electron microscopy (SEM), atomic force microscopy (AFM), nanoparticle tracking analysis system (NTA), and small-angle neutron scattering (SANS) to provide information on the particle size of nanoformulations.
Our Workflow of Nanoformulation Mean Particle Size Distribution and Testing
CD Formulation can quickly respond to your detailed requirements and provide the mean particle size distribution and testing services according to our robust project management process and our advanced equipment.
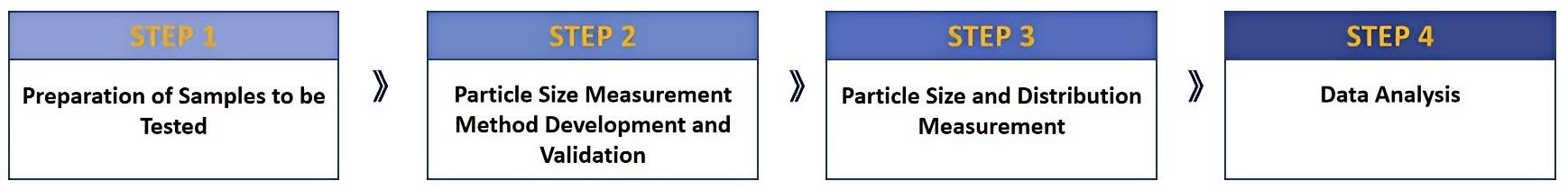 Fig.1 Our workflow of mean particle size distribution and testing. (CD Formulation)
Fig.1 Our workflow of mean particle size distribution and testing. (CD Formulation)
Why Choose Us for Nanoformulation Mean Particle Size Distribution and Testing?
- We hold advanced instruments and equipment, such as laser scattering particle size analyzer for mean particle size distribution and testing of APIs, nanocarriers, and nanoformulations.
- Our team is a strong, experienced and elite team of experts, and they can quickly respond to your detailed requirements and offer suitable analytical proposal for your request about mean particle size distribution and testing immediately.
- Our sample circulation cycle is short, and we can expeditiously arrange testing according to the testing nature of your samples, helping you quickly obtain test reports.
Published Data
Technology: Asymmetric flow field flow fractionation measurement method
Journal: Molecular Pharmaceutics
IF: 4.9
Published: 2019
Results:
The authors evaluated the use of asymmetric flow field flow fractionation in conjunction with a multi-angle light scattering and dynamic light scattering detector (AF4-MALS-DLS) as an alternative to batch-mode DLS for measuring the physical properties of lipid-based nanoparticles. While batch-mode DLS can be used as a quick and easy method to provide an initial understanding of sample integrity and polydispersity, it is not suitable for resolving subtle changes in particle size distribution. Meanwhile, the nanoparticle sorting technology introduced by the authors through flow field fractionation technology combined with online DLS and MALS can evaluate batch-to-batch variability and changes in lipid nanoparticle size caused by interactions with serum proteins. This is crucial for quality control and regulatory aspects. The AF4-MALS-DLS method can be used for the preclinical characterization of lipid-based nanoparticles.
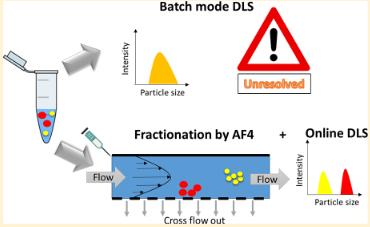 Fig.2 Asymmetric flow field flow fractionation measurement process. (Fanny Caputo, et al. 2019)
Fig.2 Asymmetric flow field flow fractionation measurement process. (Fanny Caputo, et al. 2019)
CD Formulation offers services for testing the mean particle size distribution of nanoformulations. To request testing for nanocarriers or nanoformulations, you can submit your request on our website or contact us by email or phone. We will reach out within three working days to discuss your project needs. Following our evaluation, we will send you a competitive quote. And then testing will be scheduled promptly upon your sample arrival, with a detailed test report provided afterward.
References
- Fanny Caputo, Amandine Arnould, Maria Bacia, et al. Measuring Particle Size Distribution by Asymmetric Flow Field Flow Fractionation: A Powerful Method for the Preclinical Characterization of Lipid-Based Nanoparticles. Molecular Pharmaceutics. 2019,16:756-767.
How It Works
STEP 2
We'll email you to provide your quote and confirm order details if applicable.
STEP 3
Execute the project with real-time communication, and deliver the final report promptly.
Related Services


 Fig.1 Our workflow of mean particle size distribution and testing. (CD Formulation)
Fig.1 Our workflow of mean particle size distribution and testing. (CD Formulation) Fig.2 Asymmetric flow field flow fractionation measurement process. (Fanny Caputo, et al. 2019)
Fig.2 Asymmetric flow field flow fractionation measurement process. (Fanny Caputo, et al. 2019)
