Nanoformulation Residual Solvent Testing Service
Inquiry
Residual solvents are critical in the fabrication of nanoformulations. However, residual solvents may pose health risks and affect the quality and safety of the final nanoformulation product. Residual solvent testing is a strategy to detect and eliminate these contaminants, ensuring product safety and quality by controlling residual solvents. CD Formulation can determine residual solvents of nanoformulations to meet customers' different quality requirements, including consultation on residual solvent determination, screening of residual solvents, residual solvent testing method development and validation.
Classification of Residual Solvents
Residual solvents are divided into four different toxicity classes regarding their dangerousness (toxicity).
Class 1 Residual Solvents
Class 1 residual solvents should be avoided and include known human carcinogens, or substances that are suspected of causing cancer, as well as environmental hazards. Class 1 residual solvents are controlled by concentration limits (ppm).
| Class 1 Residual Solvents |
Concentration Limit (ppm) |
Concern |
| Benzene |
2 |
Carcinogen |
| Carbon Tetrachloride |
4 |
Toxic and environmental hazard |
| 1,2-Dichloroethane |
5 |
Toxic |
| 1,1-Dichloroethene |
8 |
Toxic |
| 1,1,1-Trichloroethane |
1500 |
Environmental hazard |
Class 2 Residual Solvents
Class 2 residual solvents should be restricted in use, including non-genotoxic carcinogens, solvents that may cause irreversible toxicity such as neurotoxicity or distortion, and solvents that may have severe but reversible toxicity. Class 2 residual solvents generally use PDE (permissible daily exposure) or concentration limit control.
| Class 2 Residual Solvents |
PDE (mg/day) |
Concentration Limit (ppm) |
| Xylene |
21.7 |
2170 |
| Acetonitrile |
4.1 |
410 |
| Chlorobenzene |
3.6 |
360 |
| Chloroform |
0.6 |
60 |
| Cyclohexane |
38.8 |
3880 |
| 1,2-Dichloroethene |
18.7 |
1870 |
| 1,2-Dimethoxyethane |
1.0 |
100 |
| N,N-Dimethylacetamide |
10.9 |
1090 |
| N,N-Dimethylformamide |
8.8 |
880 |
| 1,4-Dioxane |
3.8 |
380 |
| 2-Ethoxyethanol |
1.6 |
160 |
| Ethylene Glycol |
6.2 |
620 |
| Formamide |
2.2 |
220 |
| Hexane |
2.9 |
290 |
| Methanol |
30.0 |
3000 |
| 2-Methoxyethanol |
0.5 |
50 |
| Methylbutylketone |
0.5 |
50 |
| Methylcyclohexane |
11.8 |
1180 |
| Methylene Chloride |
6.0 |
600 |
| N-Methylpyrrolidone |
5.3 |
530 |
| Nitromethane |
0.5 |
50 |
| Pyridine |
2.0 |
200 |
| Sulfolane |
1.6 |
160 |
| Tetrahydrofuran |
7.2 |
720 |
| Tetralin |
1.0 |
100 |
| Toluene |
8.9 |
890 |
| Trichloroethylene |
0.8 |
80 |
Class 3 Residual Solvents
Class 3 residual solvents have low potential toxicity. There is no need to establish a health-based exposure level. They should be limited by GMP or other quality-based requirements. The PDE of Class 3 residual solvents must be 50 mg or less per day.
- Acetic acid
- Acetone
- Anisole
- 1-Butanol
- 2-Butanol
- Butyl acetate
- Tert-Butylmethyl ether
- Cumene
- Dimethyl sulfoxide
- Ethanol
- Ethyl acetate
- Ethyl ether
- Ethyl formate
- Formic acid
- Heptane
- Isobutyl acetate
- Isopropyl acetate
- Methyl acetate
- 3-Methyl-1-butanol
- Methylethylketone
- Methylisobutylketone
- 2-Methyl-1-propanol
- Pentane
- 1-Pentanol
- 1-Propanol
- 2-Propanol
- Propyl acetate
Class 4 Residual Solvents
Class 4 residual solvents are unknown toxicity, there is insufficient toxicity information, and less solvents are used. If this type of solvent is used, the rationality needs to be demonstrated.
- 1,1-diethoxypropane
- Methyl isopropyl ketone
- 1,1-Dimethoxymethane
- Methyltetrahydrofuran
- 2,2-Dimethoxypropane
- Petroleum ether
- Isooctane
- Trichloroacetic acid
- Isopropyl ether
- Trifluoroacetate
Our Residual Solvent Testing Service
When testing residual solvents in nanoformulation, we must first clarify which solvents may remain, that is, where the solvent may be brought in. Possible residual solvents need to be considered in all aspects from raw materials, nanoformulation preparation processes, and prescriptions. And then determine the residual amount limit of the residual solvent according to the type of residual solvent. Our R&D team can quick evaluate your residual solvent testing requirements and provide you with our professional proposal including but not limit to confirming residual solvents which to be tested, selecting residual solvent testing techniques, analytical method development and validation.
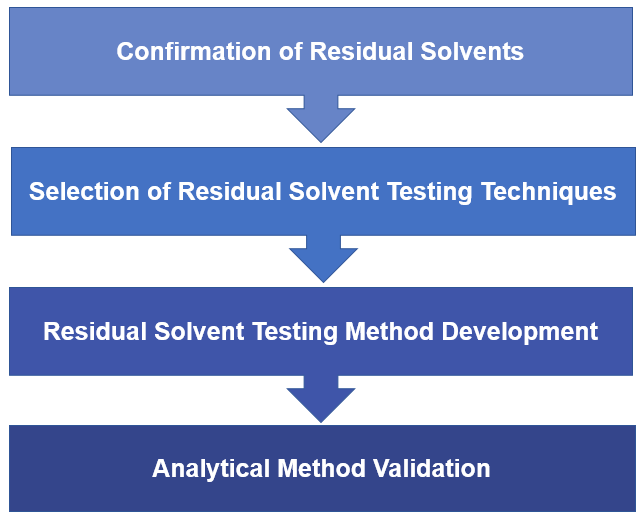 Fig.1 Our workflow of residual solvent testing. (CD Formulation)
Fig.1 Our workflow of residual solvent testing. (CD Formulation)
Why Choose Us to Test Residual Solvent?
- We have multiple gas chromatography instruments and other advanced equipment for testing residual solvents.
- Our R&D technical team have rich experience in residual solvent testing, analytical method development and validation, and provide you with residual solvent testing services immediately.
- We also can offer our special residual solvent testing proposal and our professional after-sale technical support services.
Published Data
Technology: Headspace GC-FID Technique
Journal: Journal of Drug Delivery Science and Technology
IF: 5
Published: 2020
Results:
The authors investigated the use of supercritical carbon dioxide (scCO2) to eliminate the residual organic solvent dichloromethane (DCM) from risperidone-containing microspheres. The authors used a conventional emulsion solvent evaporation procedure to prepare the microspheres, and scCO eliminated residual DCM. To obtain the maximum % elimination rate, the authors employed a central composite design (CCD) with static time, dynamic time, pressure and temperature parameters. After scCO2 treatment, the authors used headspace GC-FID to analyze residual DCM. The authors also studied the characterization, encapsulation efficiency, drug loading and in vitro release of the microspheres. Studies have shown that ScCO2 technology can increase porosity and remove DCM, thereby enhancing uniform distribution of drugs in the matrix. The microspheres before scCO2 treatment contain a large amount of solvent, which can enhance the mobility of the polymer chains and thereby enhance the release of the drug. Therefore, supercritical carbon dioxide (scCO2) can lead to sustained release of drugs from the microsphere matrix.
CD Formulation has a team with rich experience to provide residual solvent testing services for your nanoformulations during both your nanoformulation development and quality control. If you are interested in our residual solvent testing services, please kindly contact us for detailed information and communication.
References
- ICH Q3C: Impurities: Guideline for Residual Solvents.
- Hossein Kamali, Mohsen Atamanesh, Ehsan Kaffash, et al. Elimination of residual solvent from PLGA microspheres containing risperidone using supercritical carbon dioxide. Journal of Drug Delivery Science and Technology. 2020,57,101702.
How It Works
STEP 2
We'll email you to provide your quote and confirm order details if applicable.
STEP 3
Execute the project with real-time communication, and deliver the final report promptly.
Related Services


 Fig.1 Our workflow of residual solvent testing. (CD Formulation)
Fig.1 Our workflow of residual solvent testing. (CD Formulation)
