Nanoformulation Stability Testing Service
Inquiry
For all new nanomedicines, stability testing should include testing of all parameters that are susceptible to change during transportation and storage and that may affect the safety, efficacy, and quality of these products. CD Formulation has established a unique and professional nanoformulation stability testing strategy based on ICH guidelines and our nanoformulation analytical platform. We can quickly respond to researchers’ needs for nanoformulation stability testing and offer systematic, comprehensive, and regulatory-compliant stability study protocols and reports.
Why is Nanoformulation Stability Testing Needed?
It is important to establish appropriate methods to accurately evaluate the stability of nanoformulations. Factors that may affect the stability of nanomedicines include degradation of polymers or nanoparticles, aggregation of nanoparticles, degradation of drugs, leakage of drugs in the carrier, degradation of surface modification molecules or coating materials, etc. Sometimes it is difficult to fully evaluate the stability of nanoparticles through simple particle size and surface charge measurements. It is necessary for us to combine the characteristics of nanomedicines to establish and research stability evaluation methods or indicators that meet nanoformulation quality control requirements.
Our Nanoformulation Stability Testing Services
Research on the stability of nanoformulations includes the investigation of stability during storage, preparation stage and clinical use, as well as the influencing factors. We determine the shelf life of nanoformulations and recommend appropriate storage conditions by evaluating the thermal stability and sensitivity to moisture of nanoformulations under shipping and storage conditions (long-term and accelerated).
Accelerate and Long-term Stability Testing
Accelerated thermal stability testing involves the use of higher temperatures for rapid reactions that can be extrapolated to required storage conditions. In general, we recommend long-term and accelerated storage conditions of 25℃ ± 2℃/60% RH ± 5%RH and 40℃ ± 2℃/75% RH ± 5% RH, respectively according to ICH guideline Q1A. If appropriate, intermediate storage conditions (30℃ ± 2℃/65% RH ± 5% RH) are recommended.
Photostability Testing
According to ICH guideline Q1B, photostability testing is recommended for drug products containing photosensitive drugs.
In addition, if any nanoformulations are intended to be stored in a refrigerator, we will recommend long-term and accelerated storage conditions of 5℃± 3℃and 25℃± 2℃/60% RH ±5% RH, respectively. If any nanoformulations are intended to be stored in a freezer, only long-term storage conditions of -20℃ ± 5℃re recommended.
Our Nanoformulation Stability Testing Indicators
We have gained extensive hands-on experience in conducting stability testing and research on nanoformulation. Our stability research on nanoformulation encompasses evaluating various indicators and monitoring their influencing factors.
- Particle size and distribution, particle shape and charge.
- Dispersion state of drugs or nanoparticles.
- Nanoparticle redispersibility.
- In vitro dissolution, release or leakage of drugs.
- Degradation of nanoparticles (including removal or exchange of surface ligands).
- Compatibility of nanoparticles and packaging materials.
- Compatibility with diluents, syringes, infusion bags, etc. during preparation and use.
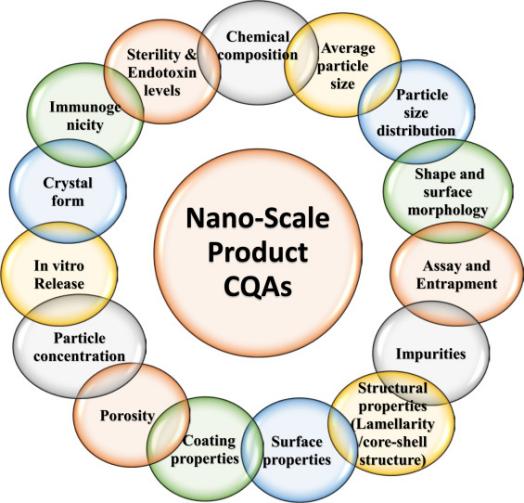 Fig.1 The Nano-scale product COA. (Akash Chaurasiya, et al. 2022)
Fig.1 The Nano-scale product COA. (Akash Chaurasiya, et al. 2022)
Our Workflow of Nanoformulation Stability Testing
Our team can quickly respond to your nanoformulation stability testing requirements, and you can get the test report in just following six steps.
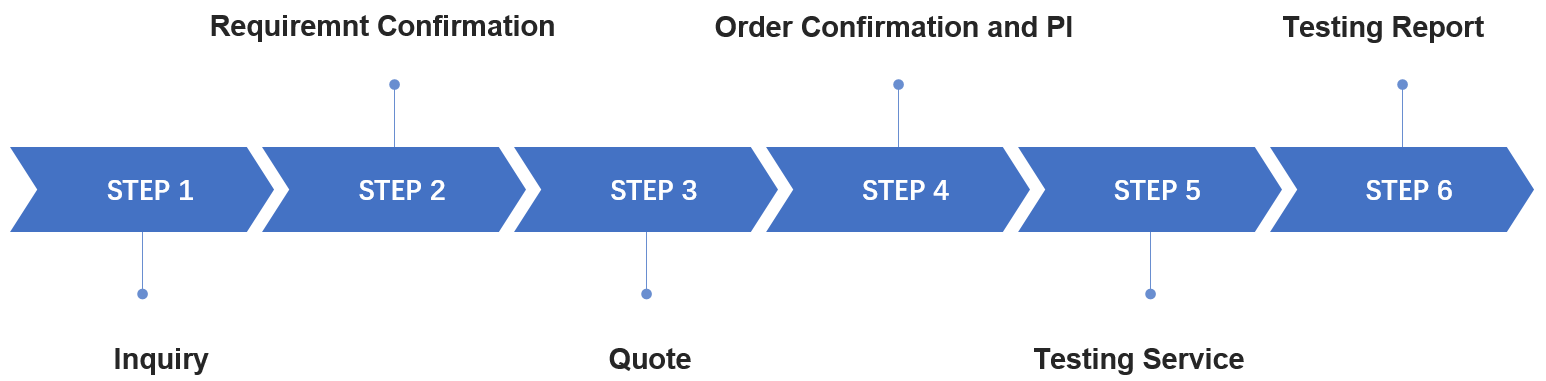 Fig.2 Our workflow of nanoformulation stability testing. (CD Formulation)
Fig.2 Our workflow of nanoformulation stability testing. (CD Formulation)
Why Choose Us to Test Nanoformulation Stability?
- We have multiple nanoformulation analytical platforms, such as nanoproperty characterization technique platform, and advanced instruments and equipment. With our strong and powerful hardware environment, we have the ability to provide you with stability research and testing projects on nanoformulations.
- We have a professional technical team with backgrounds in pharmacy, biology, chemistry, materials science and other disciplines. With many years of experience in nanoformulation research and development, they have profound knowledge in the formulation development and quality research of nanoformulations.
- Our nanoformulation stability testing service process is relatively simple, which helps to quickly confirm your project needs and your sample information and provide you with our high-quality stability experimental proposal and report in a timely manner.
Published Data
Technology: UV-Vis spectroscopy technique
Journal: International Journal of Nanomedicine
IF: 8
Published: 2021
Results:
The authors summarize some key approaches to the preparation of highly stable gold nanoparticles using polymers, including heterocyclic species, polyethylene glycols, amines, and thiols, as well as advances in natural product gold nanoparticles. At the same time, the authors summarized the stability of various Au NPs under different storage times, ionic solutions, pH, temperature, and biological matrices. Finally, the author focuses on the applications of Au NPs in the field of nanomedicine, such as drug delivery, bioimaging, photothermal therapy (PTT), clinical diagnosis, nanozymes, and radiotherapy (RT). This research has important implications for the application of gold nanomaterials in fields such as nanomedicine and biosensors.
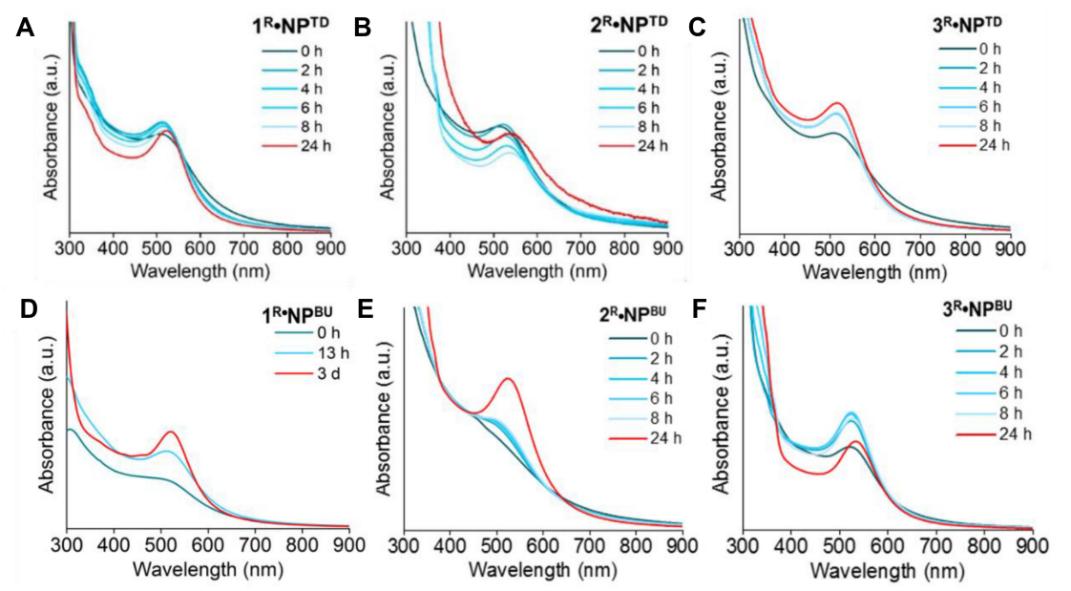 Fig.3 Stability studies of NHC-Au NPs . (Minwei Zhang, et al. 2021)
Fig.3 Stability studies of NHC-Au NPs . (Minwei Zhang, et al. 2021)
CD Formulation has developed an advanced nanoformulation analytical technology platform and utilizes a skilled technical team to offer efficient and high-quality nanoformulation stability research and testing services to researchers and pharmaceutical companies. Additionally, it supports global researchers in advancing the development of nanoformulations to a more advanced stage. If you are interested in what we offer, please feel free to contact us for more information.
References
- ICH Q1A(R2) Stability Testing of New Drug Substances and Products
- ICH Q1B Stability Testing Photostability Testing of New Drug Substances and Products.
- Akash Chaurasiya, Amruta Gorajiya, Jayabalan Nirmal. Chapter 19 - Stability testing parameters of nanoscaled product development. Multifunctional Nanocarriers. 2022, 475-500.
- Minwei Zhang, Shuxuan Shao, Haitao Yue, et al. High Stability Au NPs: From Design to Application in Nanomedicine. International Journal of Nanomedicine. 2021,16: 6067-6094.
How It Works
STEP 2
We'll email you to provide your quote and confirm order details if applicable.
STEP 3
Execute the project with real-time communication, and deliver the final report promptly.
Related Services


 Fig.1 The Nano-scale product COA. (Akash Chaurasiya, et al. 2022)
Fig.1 The Nano-scale product COA. (Akash Chaurasiya, et al. 2022) Fig.2 Our workflow of nanoformulation stability testing. (CD Formulation)
Fig.2 Our workflow of nanoformulation stability testing. (CD Formulation) Fig.3 Stability studies of NHC-Au NPs . (Minwei Zhang, et al. 2021)
Fig.3 Stability studies of NHC-Au NPs . (Minwei Zhang, et al. 2021)
