Nanoformulation Analytical Method Development and Optimization
Inquiry
Developing and optimizing analytical methods for nanoformulations is crucial for ensuring thorough quality control research on nanomedicines and nanocarrier materials. With our extensive knowledge and background in creating analytical methods for various nanoformulations such as nanoparticles, liposomes, exosomes, nanoemulsions, and nanogels, CD Formulation offers tailored services for nanoformulation analytical method development and optimization to support development and quality control efforts.
Why Develop and Optimize Nanoformulation Analytical Method?
Developing and perfecting analytical methods for nanoformulations is a critical component of nanomedicine research and development. This process entails establishing a set of procedures to thoroughly examine the physical, chemical, and biological attributes of the medication. This is crucial to ensure that the nanomedicine adheres to quality standards and regulatory guidelines. By enhancing and fine-tuning analysis methods, scientists can guarantee the reliability and accuracy of analysis results, providing them with precise data to improve their understanding of drug properties. This, in turn, helps guide future nanomedicine research and progress. The process of developing and optimizing analytical methods typically involves selecting, refining, validating, and implementing analytical techniques during drug development and production stages, especially for quality control in nanomedicine, analyzing active ingredients, testing purity, and evaluating in vitro release, among other factors.
Our Nanoformulation Analytical Method Development and Optimization Process
CD Formulation has been committed to nanoformulation analytical method development and optimization for years. And we can provide customers with our professional nanoformulation analytical method development and optimization for the following indicators.
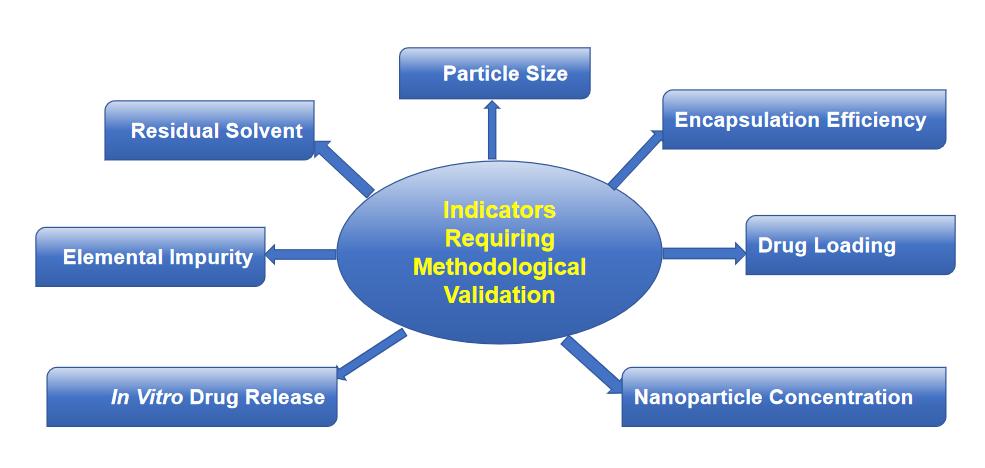 Fig.1 Indicators requiring nanoformulation methodological validation. (CD Formulation)
Fig.1 Indicators requiring nanoformulation methodological validation. (CD Formulation)
Particle Size Testing Method Development and Optimization
Initially, we select the appropriate particle size measurement method based on the characteristics of the nanomaterial to be tested. We then optimize the response parameters by adjusting the instrument settings to ensure that the analysis method used is the most effective.
Nanoformulation Encapsulation Efficiency Testing Method Development and Optimization
Our encapsulation determination methods include size exclusion chromatography, microcolumn centrifugation, NMR, dialysis, ion exchange chromatography, etc. At first, we need to select the appropriate nanoformulation encapsulation efficiency testing method, and then optimize the testing method by adjusting the instrument parameters.
Nanoparticle Concentration Testing Method Development and Optimization
We have established nanoparticle concentration testing methods, such as dynamic light scattering (DLS), nanoparticle tracking analysis (NTA) and resistive pulse sensing and then optimize the instrument parameters in order to gain more robust nanoparticle concentration testing approaches.
In Vitro Drug Release Testing Method Development and Optimization
We have a strong focus on developing appropriate methods to evaluate the in vitro drug release of nanoformulations, such as sample and separate, membrane-free dissolution, continuous flow, dialysis assay, etc. Then we optimize in vitro drug release testing method based on the proprieties of each method.
Nanoformulation Elemental Impurity Testing Method Development and Optimization
Our techniques for nanoformulation elemental impurities cover ultraviolet spectrophotometry (UV), atomic absorption spectrophotometry (AAS), X-ray fluorescence spectrometry (XRF), and inductively coupled plasma emission spectrometry (ICP-OES) and inductively coupled plasma mass spectrometry (ICP-MS). Firstly, we select the appropriate nanoformulation element impurity testing method, and then adjust the instrument parameters to gain a more reliable and accurate testing method for nanoformulation element impurities.
Nanoformulation Residual Solvent Testing Method Development and Optimization
The residual solvents of most nanoformulations are determined by gas chromatography. We first develop the nanoformulation residual solvent testing method by gas chromatography and then optimize chromatographic conditions by adjusting the instrument parameters to ensure that the nanoformulation residual solvent testing method used is optimal.
Why Choose Us for Nanoformulation Analytical Method Development and Optimization?
- Relying on our world-class and advantaged instruments and equipment, we can offer nanoformulation analytical method development and optimization services.
- Our core team has rich experience in nanoformulation analytical method development and optimization, including particle size, nanoparticle concentration, in vitro drug release, etc.
- We can respond to your detailed requirements for nanoformulation analytical method development and optimization immediately as soon as you reach out to us and let us know your detailed project.
Published Data
Technology: RP-HPLC method development and validation
Journal: Journal of Chromatography B
IF: 3.0
Published: 2022
Results:
The authors developed an accurate, precise, selective, and sensitive reversed-phase high-performance liquid chromatography (RP-HPLC) method for the simultaneous determination of the two compounds in nanoformulations and glioma cells, optimized the chromatographic conditions, and then used the optimized analytical method to determine the retention time of resveratrol and gefitinib. The method was shown to be specific, precise (RSD 2%), and accurate (>90%) for the analysis of resveratrol and gefitinib in the presence of PHLNPs. Simultaneous analytical methods were also successfully developed to determine the percentage drug encapsulation efficiency, percentage drug loading of resveratrol and gefitinib in PHLNPs, as well as secondary estimates for in vitro drug release profiles and percentage cellular uptake studies. The results showed that the developed analytical method can simultaneously detect and quantify these drugs in other nanoformulations and in vivo studies.
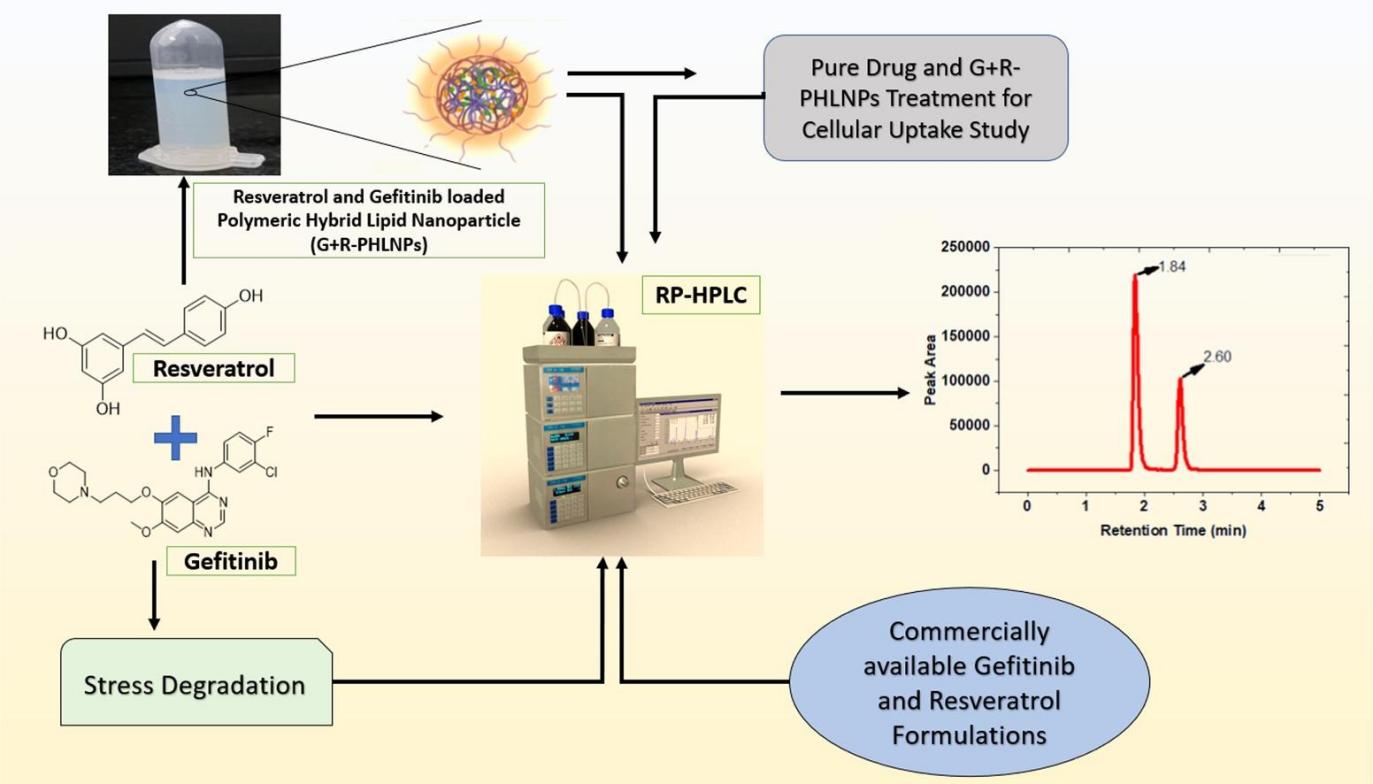 Fig.2 RP-HPLC method development and validation for simultaneous quantification of gefitinib and resveratrol in polymeric hybrid lipid nanoparticles and glioma cells. (P Sathishbabu, et al. 2022)
Fig.2 RP-HPLC method development and validation for simultaneous quantification of gefitinib and resveratrol in polymeric hybrid lipid nanoparticles and glioma cells. (P Sathishbabu, et al. 2022)
As a leading company in nanoformulation development and manufacturing, CD Formulation has a range of advanced analytical tools and a skilled team with extensive experience to offer services in developing and optimizing analytical methods for nanoformulations. If you have inquiries regarding this service, please reach out to us for more information.
References
- P Sathishbabu, Umme Hani, C Shakeela, et al. A novel RP-HPLC method development and validation for simultaneous quantification of gefitinib and resveratrol in polymeric hybrid lipid nanoparticles and glioma cells. Journal of Chromatography B. 2022, 1212:123483.
How It Works
STEP 2
We'll email you to provide your quote and confirm order details if applicable.
STEP 3
Execute the project with real-time communication, and deliver the final report promptly.
Related Services


 Fig.1 Indicators requiring nanoformulation methodological validation. (CD Formulation)
Fig.1 Indicators requiring nanoformulation methodological validation. (CD Formulation) Fig.2 RP-HPLC method development and validation for simultaneous quantification of gefitinib and resveratrol in polymeric hybrid lipid nanoparticles and glioma cells. (P Sathishbabu, et al. 2022)
Fig.2 RP-HPLC method development and validation for simultaneous quantification of gefitinib and resveratrol in polymeric hybrid lipid nanoparticles and glioma cells. (P Sathishbabu, et al. 2022)
