Oral Thin Film Manufacturing Technologies
Inquiry
The oral thin film is being looked upon as a novel approach to designing efficient drug delivery systems. Extensive research is being done to enable different types of drugs to be formulated into these strips and to overcome specific challenges confronted during manufacture, scale-up, and the cost-effectiveness of the oral thin film. CD Formulation provides contract manufacturing services for oral thin film, leveraging expertise in various manufacturing technologies such as solvent casting technology, hot melt extrusion technology, semi-solid casting technology, solid dispersion extrusion technology, rolling technology, 3D printing technology, etc.
Formulation of Oral Thin Films
Many factors are considered when manufacturing oral thin films. Different attributes include masking the taste, quick-dissolving, maintenance of medicinal properties, physical appearance, and acceptable mouth feel. Some components required in manufacturing oral thin film include active pharmaceutical ingredients, film-forming polymers, plasticizers, surfactants, sweetening agents, saliva-stimulating agents, super disintegrants, coloring agents, and flavoring agents. Therefore, we will choose the most suitable manufacturing technology for the production process of oral thin film.
As an innovative drug delivery method, oral thin film has many advantages, such as not requiring water at the disposal, no risk of suffocation, increased stability compared with liquid form, but there are also some limitations. Our professional technical team is familiar with the characteristics of various manufacturing technologies and dares to innovate. Based on these limitations of oral dissolved film, we develop corresponding technical methods to provide professional guidance for manufacturing oral thin films.
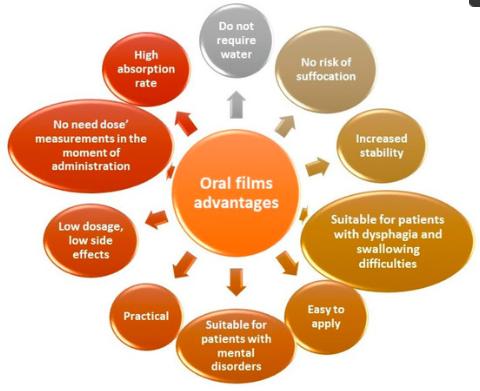 Fig.1 Advantages of Oral Thin Films. (Emma Adriana Ozon, et al., 2023)
Fig.1 Advantages of Oral Thin Films. (Emma Adriana Ozon, et al., 2023)
Our Oral Thin Film Manufacturing Technologies
As experts in the field of oral thin film drug delivery, CD Formulation has specialized oral thin film manufacturing technologies, including but not limited to:
Solvent casting technology is the most generally utilized method to prepare oral thin film because of its simple preparation, low processing cost, and ease of application. The obtained solution is incorporated with the API dissolved in suitable solvents. The entrapped air is removed by vacuum. The resulting solution is cast as a film and allowed to dry, then cut into pieces of the desired size.
Hot melt extrusion technology refers to mixing the API and other excipients in a dry state. After the heating process begins, the molten mass is extruded from the hot melt extruder, and the film is cooled and then cut to the desired size. The advantage of this method is that no solvent is required at all.
In the semisolid-casting technology, first, a solution of the water-soluble, film-forming polymer is prepared, and the solution is added to a solution of acid-insoluble polymer. Then, an appropriate amount of plasticizer is added to get a gel group, and the prepared gels are poured into films or strips using a controlled heat source.
Solid dispersion refers to the dispersion of one or more APIs in an inert carrier in a solid state in the presence of amorphous hydrophilic polymers using methods such as HME. In solid-dispersion extrusion, immiscible components are extruded with drugs, and solid dispersions are prepared. The solid dispersions are shaped into films using dies.
A solution or suspension containing the drug is rolled on a carrier using rolling technology. The solvent is mainly water and a mixture of water and alcohol. The film is dried on rollers and cut into desired sizes and shapes.
3D printing technology can solve the formulation problems of producing oral thin films. Currently, buccal dosage forms are reserved for potent agents due to the limited capacity of drug incorporation. 3D printing technology could be used to superimpose the layers of oral thin film to accommodate more active ingredients per unit area. In addition, 3D printing can provide a platform for long-term control of drug release, reducing the frequency of administration.
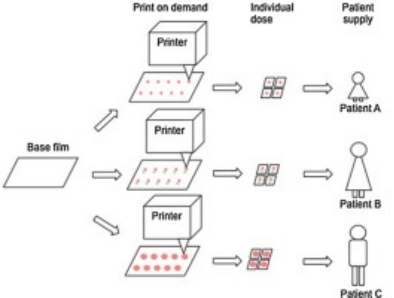 Fig.2 Printing technologies for oral film formulations. (Maren Preis, et al., 2023)
Fig.2 Printing technologies for oral film formulations. (Maren Preis, et al., 2023)
Explore Our Oral Thin Film Manufacturing
CD Formulation provides customers with CGMP manufacturing services from laboratory scale to commercial scale, and we have advanced equipment and strict quality control systems to ensure that we can quickly bring our customers' oral thin film products to the market.
Pilot Scale-up Manufacturing Services
We provide comprehensive pilot scale-up manufacturing services for oral thin films and have different manufacturing methods to meet the pilot scale-up of various oral thin films.
Preclinical Manufacturing Services
Our team consists of experts with extensive experience in oral thin film formulation and manufacturing who can accurately solve any preclinical problems and guide your commercial production.
Commercial Manufacturing Services
We can provide guidance and support throughout the oral thin film product development process, from formulation and testing to scale-up and commercialization.
Advantages of Our Oral Thin Film Manufacturing Technologies
- We can customize dosages and formulations to meet the specific research needs.
- Our manufacturing process allows for the quick development and production of oral thin films, enabling faster market entry for new drugs or reformulated products.
- We constantly prioritize strict compliance with worldwide standards in manufacturing, quality, safety and environment, ensuring that the oral thin films produced meet regulatory requirements and high-quality standards.
- We manage an end-to-end supply chain, including contractor supply chain management.
Published Data
Technology: Manufacturing Techniques of Orally Dissolving Films
Journal: Pharmaceutical Technology
IF: 0.1
Published: 2011
Results: The authors discuss these methods and the various parameters for evaluating dissolving films. One or a combination of the following processes can manufacture ODFs: solvent casting, semi-solid casting, hot-melt extrusion (HME), solid-dispersion extrusion, and rolling. The most commonly used methods of film manufacturing are solvent casting and HME.
CD Formulation is equipped with a wide range of high-performance equipment for the contract production of oral thin film. As a contract development and manufacturing organization, we handle the entire supply chain, including purchasing, mixing, manufacturing, packaging, and storage. If you require our oral thin film manufacturing technologies services, please contact us by phone or email, and our colleagues will get back to you within three working days.
References
- Maren Preis, Joerg Breitkreutz, et al. Perspective: Concepts of printing technologies for oral film formulations. International Journal of Pharmaceutics. 2015, Vol (494):578-584.
- Emma Adriana Ozon, Iulian Sarbu, et al. Three-Dimensional Printing Technologies in Oral Films Manufacturing—A Minireview. Processes. 2023, 11(9): 2628.
How It Works
STEP 2
We'll email you to provide your quote and confirm order details if applicable.
STEP 3
Execute the project with real-time communication, and deliver the final report promptly.
Related Services

 Fig.1 Advantages of Oral Thin Films. (Emma Adriana Ozon, et al., 2023)
Fig.1 Advantages of Oral Thin Films. (Emma Adriana Ozon, et al., 2023) Fig.2 Printing technologies for oral film formulations. (Maren Preis, et al., 2023)
Fig.2 Printing technologies for oral film formulations. (Maren Preis, et al., 2023)
