Liposome Analysis and Characterization
Inquiry
Liposomes are widely recognized as a prominent drug delivery system due to their remarkable versatility and ability to offer protection for encapsulated drugs, thereby enhancing stability and preventing degradation. To ensure the optimal functionality of liposomes as carriers of bioactive substances, it is imperative to thoroughly characterize their properties, including size, layering, surface charge, quantitative composition, and encapsulation rate. Common techniques employed for the analysis and characterization of liposomes encompass 31P nuclear magnetic resonance (NMR) (utilized for studying membrane fluidity and thermal phase transitions), dynamic light scattering (DLS), atomic force microscopy (AFM), fluorescence spectroscopy, high-performance liquid chromatography (HPLC), capillary electrophoresis (CE), among others. CD Formulation employs diverse methodologies to assist customers in characterizing liposomal solutions and drug-carrying liposomes.
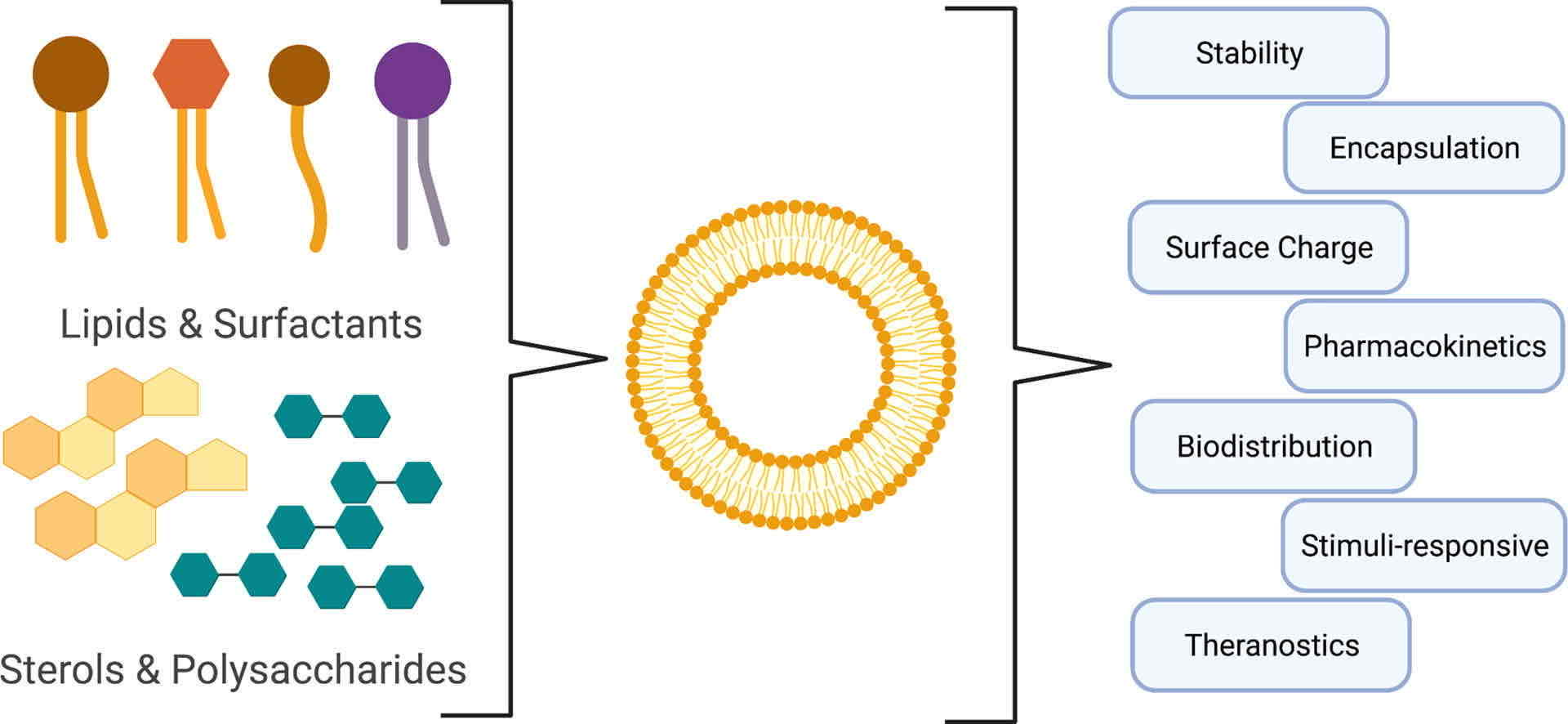 Fig.1 Liposome Analysis and Characterization. (Danielle E. Large, et al., 2021)
Fig.1 Liposome Analysis and Characterization. (Danielle E. Large, et al., 2021)
The Importance of Liposome Analysis and Characterization
The properties specific to liposome-based drug products, such as their composition, size, and preparation techniques, are crucial quality attributes. Physicochemical properties like size distribution and morphology also play a significant role in determining the overall stability and efficacy of liposomes as drug carriers. Other key attributes include payload, encapsulation efficiency, bilayer structure, and biological characteristics. Accurate measurement of these parameters is critical for early drug development and quality control of new products. Furthermore, these parameters greatly influence the bioactivity and biodistribution of liposome-based drug and gene delivery platforms. Therefore, employing analytical techniques that provide objective and reliable information about the characteristics of liposome particles can significantly enhance the product development process while reducing time required for ensuring final quality.
Our Services for Liposome Analysis and Characterization
The study of liposome quality characterization and critical quality attributes (CQAs) can be utilized for formulation process screening and stability testing, as well as serving as a reference and foundation for subsequent non-clinical research leading to clinical application. CD Formulation, a specialized nanoformulation service company, focuses on the development, manufacturing, analysis, and evaluation of liposome formulations. With extensive experience in liposome development, analysis, and evaluation spanning many years, our services encompass various aspects of liposomal characterization including but not limited to:
| Items |
Specific Analytical Services |
| Universal Liposome Characterization |
Liposome Size Distribution Testing |
| Liposome Zeta Potential Testing |
| Encapsulation Efficiency (EE%) Testing |
| Liposome Drug Loading Testing |
| Thermodynamic Properties Analysis of Liposome Membranes |
| Liposome Morphology/Structure Analysis |
| Liposome Membrane Permeability Testing |
| Liposome Lamellarity Analysis |
| Liposome Phase Transition Temperature Analysis |
| Liposome Biological Evaluation |
Liposome In Vitro Release Study |
| Liposome Cellular Uptake Study |
| Liposome Pharmacokinetics Study |
| Liposome Biodistribution Study |
| Liposome In Vitro Bioactivity Evaluation |
| Liposome Drug Efficacy Evaluation |
| Oral Liposome Characterization |
The main focus of this service is to investigate the release of oral liposomes in simulated saliva, gastric juice, and intestinal fluid. |
| Transdermal Liposome Characterization |
Liposome In Vitro Transdermal Diffusion Study |
| Liposome In Vivo Skin Permeation and Accumulation Study |
| Transdermal Liposome Skin Irritation Testing |
| Injectable Liposome Characterization |
Injectable Liposome Particulate Matter Analysis |
| Injectable Liposome Sterility and Endotoxin Testing |
| Injectable Liposome Hemolysis Testing |
| Liposome Aerosol Characterization |
Atomization rate |
| Aerosol droplet size distribution |
| Size of liposome vesicle after atomization |
| Encapsulation rate of the encapsulated drug after atomization |
| Lyophilized Liposome Characterization |
Lyophilized Liposome Residual Moisture Analysis |
| Lyophilized Liposome Reconstitution Analysis |
| Lyophilized Liposome Hygroscopicity Determination |
| Ocular Liposome Characterization |
Liposome Surface Tension Study |
| Liposome Corneal Permeability Assay |
| Liposome Ocular Irritation Assessment |
Our Platforms for Liposome Analysis and Characterization
| Platforms |
Specifics |
| Liposome Physicochemical Study Platform |
This platform support to offer universal analysis services such as:
- Size Distribution Testing
- Zeta Potential Testing
- Encapsulation Efficiency (EE%) Testing
- Drug Loading Testing
- Thermodynamic Properties Analysis of Membranes
- Morphology/Structure Analysis
- Membrane Permeability Testing
- Lamellarity Analysis
- Phase Transition Temperature Analysis
|
| Liposome Biological Evaluation Platform |
The platform support evaluation of biological characteristics in vitro and in vivo, such as
- in vitro release study,
- cellular uptake study,
- pharmacokinetics study,
- biodistribution study,
- in vitro bioactivity evaluation,
- drug efficacy evaluation.
|
| Oral Liposome Characterization Platform |
This platform supports in vitro pharmacodynamics studies of oral liposomes. |
| Transdermal Liposome Characterization Platform |
This platform supports transdermal liposome characterization such as:
- in vitro transdermal diffusion study,
- in vivo skin permeation and accumulation study,
- transdermal liposome skin irritation testing, etc.
|
| Injectable Liposome Characterization Platform |
This platform supports transdermal liposome characterization such as:
- particulate matter analysis,
- liposome sterility and endotoxin testing,
- hemolysis testing, etc.
|
| Liposome Aerosol Characterization Platform |
This platform supports following analyses:
- Atomization rate
- Aerosol droplet size distribution
- Size of liposome vesicle after atomization
- Encapsulation rate of the encapsulated drug after atomization
|
| Lyophilized Liposome Characterization Platform |
This platform supports following studies:
- Residual moisture analysis
- Reconstitution analysis
- Hygroscopicity determination
|
| Ocular Liposome Characterization Platform |
This platform supports following studies:
- Surface tension study
- Liposome corneal permeability assay
- Liposome ocular irritation assessment
|
Highlights for 0ur Liposome Analysis and Characterization Services
- Expertise. We have established a dedicated liposome characterization platform, and our team can support a variety of liposome characterization services, including physical and chemical properties, stability assessment, in vitro and in vivo release analysis, etc., to ensure that our customers get the most accurate and reliable results.
- Extensive and comprehensive. Our liposome characterization services support the detection and analysis of various liposome formulations, including, ophthalmic liposomes, transdermal liposomes, injectable liposomes, lyophilized liposomes and more.
- Multiple analysis methods. We can develop a variety of detection methods, and combine a variety of detection methods to verify each other, aiming to provide high sensitivity, high accuracy and high repeatability analysis results.
CD Formulation has accumulated extensive expertise and experience in the characterization of liposomes, with a specific focus on understanding the unique characteristics exhibited by different types of liposomes. Our highly skilled research team, comprising experienced professionals, has been at the forefront of this cutting-edge research for numerous years, consistently dedicated to providing comprehensive solutions tailored to our clients' needs. Should you require any assistance, please do not hesitate to contact us promptly.
References
- Danielle E. Large, Rudolf G. Abdelmessih, et al. Liposome composition in drug delivery design, synthesis, characterization, and clinical application. Advanced Drug Delivery Reviews. 2021. Volume 176.
How It Works
STEP 2
We'll email you to provide your quote and confirm order details if applicable.
STEP 3
Execute the project with real-time communication, and deliver the final report promptly.
Related Services

 Fig.1 Liposome Analysis and Characterization. (Danielle E. Large, et al., 2021)
Fig.1 Liposome Analysis and Characterization. (Danielle E. Large, et al., 2021)
