Lyophilized Liposome Characterization
Inquiry
Liposome liquid is prone to focus, fusion and drug leakage in storage. At present, the effective method is to use biopharmaceutical freeze-drying machine for vacuum freeze-drying to freeze dry liposome, reduce the hydrolysis and oxidation rate of phospholipids and drugs, and solve the instability factors. Comprehensive analytical characterization of lyophilized liposomes is essential to distinguish between stable and unstable samples. At CD Formulation, we can offer multiple analysis methods to confirm the stability of lyophilized liposomes.
Why You Need Lyophilized Liposome Characterization?
Lyophilized liposomes can be used directly as solid dosage forms such as sprays, or can be used after reconstituting liposome suspension with water or other suitable solvent hydration. However, the processes of pre-freezing, drying and rehydration are not conducive to the stability of the structure and function of liposomes. During lyophilization, the formation of ice crystals, the change of osmotic pressure, phase separation and phase transition can lead to the folding, fusion, rupture and drug leakage of the liposome membrane. Therefore, it is very important to evaluate the quality of liposomes before and after lyophilization, which is related to the stability and efficacy of liposomes before and after lyophilization.
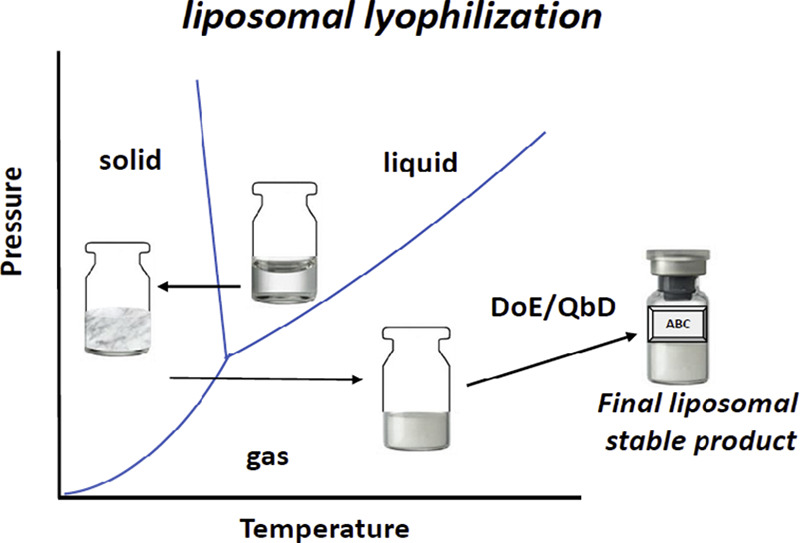 Fig.1 Schematic illustration mechanism of liposomal lyophilization. (Yuwei Wang, et al., 2019)
Fig.1 Schematic illustration mechanism of liposomal lyophilization. (Yuwei Wang, et al., 2019)
Explore Our Services for Lyophilized Liposome Characterization
For liposomes, the structural stability of the lipid carrier in the dry state, as well as the lipid bilayer recombination without leakage of the drug when water is added before and after administration, are the key features of lyophilization specific to the stability of this dosage form, so we will provide you with the characterization services of liposomes before and after lyophilization.
In order to improve the stability of product storage, it is necessary to reduce the moisture of the freeze-dried product to a very low value; For each product, to what low value: Depending on the molecule of the product, the criteria will vary. The liposomal dry powder with a water content of less than 1% is generally considered the ultimate standard. Water content serves as one of the key indicators for frozen dry liposomes, and therefore we provide testing services to ensure its accuracy.
Liposome reconstruction analysis means that lyophilized powder needs to be reconfigured during actual use, and the evaluation reconstruction analysis service we provide is related to the clinical effect of actual use. This service mainly evaluates the following parameters, such as leakage rate after reconstruction, potential distribution, and particle size distribution, which are still as the indicators before lyophilization, which are very important.
The moisture absorption of the powder refers to the phenomenon of the absorption of water on the solid surface, and the moisture absorption is related to the air state. The hygroscopic properties of lyophilized liposomes can be expressed by hygroscopic equilibrium curve or evaluated by hygroscopic weight gain.
Our Platforms for Lyophilized Liposome Characterization
We offer the following platforms and technologies for freeze-dried liposome characterization services.
|
Platforms |
Specifics |
| Hygroscopicity Analysis Platform |
- Powder dilution analysis
- Hygroscopic equilibrium curve
- Hygroscopic weighing
|
| Liposome Reconstitution Analysis Platform |
- Leakage rate detection
- Concentration analysis
- Potentiometric analysis
- Particle size analysis
|
| Liposome Residual Moisture Analysis |
- Real-time hygroscopicity analysis of freeze-drying
- Karl Fischer (KF) titration technique
- Thermogravimetric analysis (TGA) technique
- Headspace moisture analysis
|
Highlights for Lyophilized Liposome Characterization
- Cutting-edge facility. Our platforms are equipped with a wide range of analytical instruments and equipment including: High performance liquid chromatograph, particle size analyzer, moisture detector, thermogravimetric analyzer, headspace analyzer and so on.
- Strong research team. Our research team consists of a group of professional scientists who have accumulated many years of project experience in lyophilized liposome characterization services, including liposome characterization before lyophilization, lyophilized process research, lyophilized powder reconstruction, etc.
- Rapid and cost-effective. We provide cost-effective analysis services and can customize service solutions according to customer requirements. We execute and deliver experimental results quickly and quickly throughout the process
Published Data
Technology: Mucoadhesive Vehicle Based on Lyophilized Liposomes Technology
Journal: International Journal of Pharmaceutics
IF: 10.5
Published: 2022
Results: In this study, a drug carrier based on lyophilized liposomes is proposed for oral administration, aiming at systemic delivery through the sublingual mucosa. Liposomes made from egg phosphatidylcholine and cholesterol (7/3 molar ratio) are prepared and lyophilized in the presence of different additive mixtures with mucosal adhesion and masking properties. Palatability was measured in healthy volunteers. The freeze-drying cycle was optimized and the freeze-dried product was compressed to obtain round and capsule-shaped tablets and evaluated in healthy volunteers. The uniformity of weight and thickness, swelling index and liposome release of tablets were also analyzed. The results showed that the freeze-dried liposome had good palatability, small size, high comfort and good oral retention, and was suitable for sublingual administration.
 Fig.2 Aspect of lyophilized cakes inside vials (A), inside blisters (B) and after withdrawal from blister (C). (De Jesús Valle, M.J., et al., 2022)
Fig.2 Aspect of lyophilized cakes inside vials (A), inside blisters (B) and after withdrawal from blister (C). (De Jesús Valle, M.J., et al., 2022)
CD Formulation's comprehensive lyophilized liposomes characterization services are developed to offer a understanding analysis services. These services are tailored to meet the needs of researchers and developers working in the fields of pharmaceutical, biotechnology, and nanotechnology. Please do not hesitate to contact us promptly should you require any form of assistance.
References
- Yuwei Wang, David W. Grainger. Lyophilized liposome-based parenteral drug development: Reviewing complex product design strategies and current regulatory environments. Advanced Drug Delivery Reviews, 2019, Volumes 151–152,Pages 56-71.
- De Jesús Valle, M.J.; Zarzuelo Castañeda, A.; et al. Development of a Mucoadhesive Vehicle Based on Lyophilized Liposomes for Drug Delivery through the Sublingual Mucosa. Pharmaceutics. 2022, 14, 1497.
How It Works
STEP 2
We'll email you to provide your quote and confirm order details if applicable.
STEP 3
Execute the project with real-time communication, and deliver the final report promptly.
Related Services

 Fig.1 Schematic illustration mechanism of liposomal lyophilization. (Yuwei Wang, et al., 2019)
Fig.1 Schematic illustration mechanism of liposomal lyophilization. (Yuwei Wang, et al., 2019) Fig.2 Aspect of lyophilized cakes inside vials (A), inside blisters (B) and after withdrawal from blister (C). (De Jesús Valle, M.J., et al., 2022)
Fig.2 Aspect of lyophilized cakes inside vials (A), inside blisters (B) and after withdrawal from blister (C). (De Jesús Valle, M.J., et al., 2022)
