Oral Liposome Characterization
Inquiry
Liposome formulations have been utilized in certain vaccines, cancer therapeutics, and gene therapies. Currently, oral liposomes are regaining attention due to their ability to protect active substances from digestive tract instability and enhance bioavailability. CD Formulation utilizes the liposome platform to offer a variety of characterization technologies that cater to the needs of customers seeking to characterize oral liposomes.
Why You Need Oral Liposome Characterization?
Liposomal offer numerous advantages compared to most other oral formulations, such as enhanced cellular delivery and superior bioavailability and absorption. Therefore, it is crucial to thoroughly evaluate the potential of oral liposomes for their transition from research to commercial launch, particularly in terms of in vitro pharmacodynamic assessment and in vivo pharmacodynamic assessment.
Explore Our Services for Oral Liposome Characterization
Oral liposome drugs generally enter the intestine, through the liver, and then into the blood, after entering the blood there are several places: adipose tissue, lung, liver, kidney, peripheral tissue, lesion site. Our services for oral liposome characterization are as following but not limit to.
Study on the release of simulated oral fluid in vitro
In this services, the in vitro release of oral liposomes in the oral environment is evaluated by simulating an oral model in vitro.
Study on the release of in vitro simulated gastric fluid
In this service, we evaluate the in vitro release characteristics of oral liposomes into the digestive tract, especially gastric juice, by simulating the human internal gastric environment. The factors affecting gastrointestinal absorption are: pH of gastric fluid, gastric emptying and gastrointestinal peristalsis, food, circulatory system, gastrointestinal secretions and physicochemical properties of drugs.
Study on the release of simulated intestinal fluid
In this service, we simulate the intestinal environment in human body to evaluate the in vitro release characteristics of oral liposomes after entering the intestine to provide reference for in vivo release and effectively link the in vivo and in vitro release.
Our Workflow of Oral Liposome Characterization
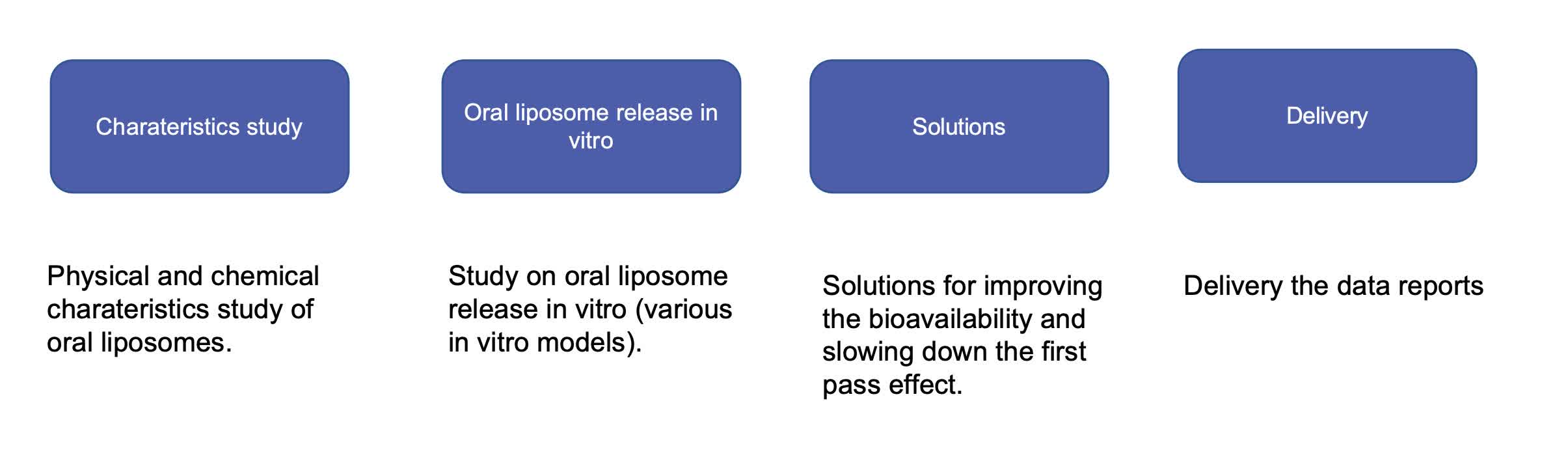 Fig.1 Our workflow for oral liposome characterization. (CD Formulation)
Fig.1 Our workflow for oral liposome characterization. (CD Formulation)
Our Platforms for Oral Liposome Characterization
| Platforms |
Specifics |
| Oral Liposome Release In Vitro Platform |
This platform support to offer oral liposome in vitro release analysis services such as:
- Oral model
- Gastric fluid model
- Intestinal model
- Liver and kidney model
|
| Oral Liposome Bioavailability Research Platform |
We collaborate with our research partner, the Research Clinic, to conduct bioavailability studies. All of our studies were randomized controlled trials (RCTS). A randomized controlled trial is a study/experiment designed to compare a "new" clinical intervention with an existing form. |
Highlights for Oral Liposome Characterization
- Expertise. Our team of experts utilizes advanced liposome platforms to provide extensive expertise in oral liposome analysis, ensuring to offer our customers the most precise and dependable results.
- Security. To sign a confidentiality agreement to protect project information and client information
- Flexible. Customizable, affordable and designed to meet the specific needs of individual customers about oral liposome analysis.
Published Data
Technology: Piperine-chitosan-coated liposomes technology
Journal: Molecules
IF: 4.6
Published: 2021
Results: In this study, the prepared preparations were evaluated using the MCF7 breast cancer cell line in physicochemical studies, mucosal adhesion studies, penetration studies, and in vitro cytotoxicity studies. Piperine liposomes (PLF) were prepared by thin film evaporation. The selected liposomes were coated with chitosan (PLFC) by electrostatic deposition to enhance mucosal adhesion and in vitro therapeutic effect. According to the findings, Prepared PPN liposomes (PLF3) and chitosan-coated PPN liposomes (PLF3C1) showed a nanoscale size range of 165.7 ± 7.4 to 243.4 ± 7.5, a narrow polydispersion index (>0.3), and a zeta potential (− 7.1 to 29.8 mV). The average encapsulation efficiency of all prepared preparations is between 60% and 80%. An overview of drug release and penetration studies shows biphasic release behavior and enhanced PPN penetration. The results of antioxidant studies in vitro showed that the antioxidant activity was comparable to that of pure PPN. Anti-cancer studies showed that the cell viability assay of tested PLF3C2 reduced IC 50 significantly (p < 0.001) compared to pure PPN. Studies have shown that oral chito-coated liposomes are a promising PPN delivery system that can improve therapeutic efficacy in breast cancer cell lines.
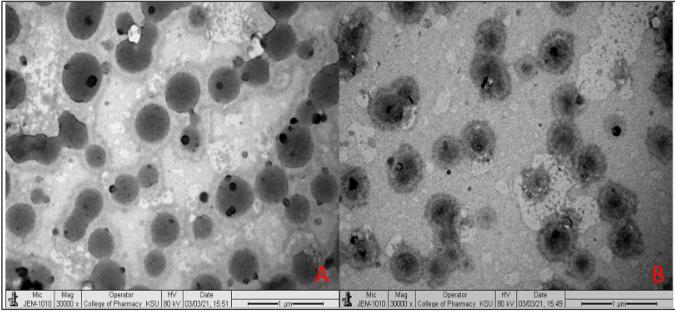 Fig.2 Surface morphology of liposomes. (Yu YF, et al., 2024)
Fig.2 Surface morphology of liposomes. (Yu YF, et al., 2024)
CD Formulation team has accumulated extensive expertise and experience in the characterization of oral liposomes. Throughout the years, our unwavering commitment has been to provide customers with tailor-made comprehensive solutions. Should you require any assistance, please do not hesitate to contact us immediately.
References
- Imam, S.S.; Alshehri, S.; et al. Formulation of Piperine-Chitosan-Coated Liposomes: Characterization and In Vitro Cytotoxic Evaluation. Molecules. 2021, 26, 3281.
How It Works
STEP 2
We'll email you to provide your quote and confirm order details if applicable.
STEP 3
Execute the project with real-time communication, and deliver the final report promptly.
Related Services


 Fig.1 Our workflow for oral liposome characterization. (CD Formulation)
Fig.1 Our workflow for oral liposome characterization. (CD Formulation) Fig.2 Surface morphology of liposomes. (Yu YF, et al., 2024)
Fig.2 Surface morphology of liposomes. (Yu YF, et al., 2024)
