Liposome Biological Evaluation
Inquiry
The CD Formulation is a renowned global pharmaceutical company that has successfully conducted numerous in vivo and in vitro animal model preparations for liposome bioassessment at our state-of-the-art liposome bioassessment center, contributing significantly to expediting the development of liposomal drugs for our esteemed clients. Our expertise lies in the skillful evaluation of drug metabolism and pharmacodynamics of liposomal drugs in a variety of diseases such as digestive system, respiratory system, cardiovascular system, nervous system and tumor.
Why You Need Liposome Biological Evaluation?
After entering the body, liposome drugs can exist in at least three forms: released as free drugs, encapsulated within liposomes, or present as empty carriers without drugs. Generally, the concentration of free drugs released in blood or normal tissues is positively correlated with adverse reactions, while the concentration of free drugs released in tumor tissues and other target organs is positively correlated with drug efficacy. The release of free drugs serves as crucial information for evaluating the efficacy and toxicity of liposomes. Furthermore, recent studies have shown that polymer materials can influence the absorption, distribution, metabolism, and excretion processes of loaded drugs, leading to changes in drug efficacy and potential adverse reactions. Polymer materials themselves may directly interact with biological macromolecules within the body; for instance, most polymer materials affect cytochrome P450 enzyme activity and some even inhibit or enhance different subtypes of this enzyme to varying degrees. Such interactions directly impact drug efficacy and may even result in the generation of toxic metabolites. Therefore, it is increasingly important to conduct comprehensive biological evaluations on liposomes.
Explore Our Services for Liposome Biological Evaluation
Our services for liposome biological evaluation are as following.
In this service, we provide in vitro release research for liposomes under different delivery routes, such as oral, ophthalmic, injectable, etc.
Our cell uptake study services provide reference value for in vivo efficacy and bioavailability of liposomes.
The pharmacokinetic study of liposome help clients link preclinical trials with clinical trials.
This service assists clients in optimizing liposome formulations, enhancing drug targeting, improving the pharmacokinetic and metabolic properties of drugs in vivo, maximizing efficacy, and minimizing the occurrence of side effects and toxicity.
The primary focus of this service is to assess the efficacy and constituents of liposome drugs, enabling customers to effectively manage the quality throughout the entire life cycle. This comprehensive evaluation plays a crucial role in stability assessment.
Our service combine actual clinical diseases and treatments, and provide customers with reasonable selection of test models and methods, and design according to drug classification and pharmacological characteristics. To provide referable in vivo analysis data and value for the development of liposome drugs.
Our Workflow of Liposome Biological Evaluation
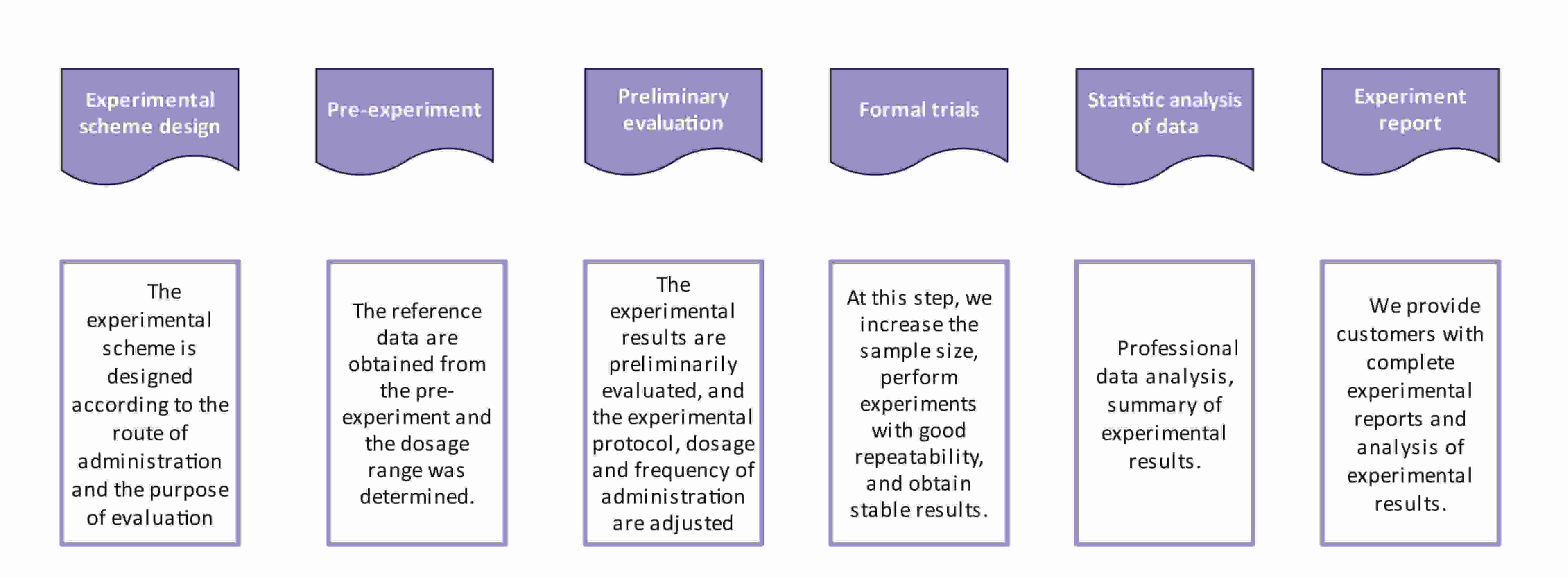 Fig.1 Workflow of liposome biological evaluation. (CD Formulation)
Fig.1 Workflow of liposome biological evaluation. (CD Formulation)
Our Platforms for Liposome Biological Evaluation
| Platforms |
Specifics |
| Liposome In Vitro Release Study Platform |
- Ability to evaluate in vitro release from multiple drug delivery routes.
- Establish a release method with good repeatability, strong operability and high standardization.
- Draw a complete liposome release curve in vitro and the end point of release.
|
| Liposome Cellular Uptake Study |
- The efficiency of cell internalization is evaluated and measured by overflow cytometry and laser confocal techonologies.
- to optimize the particle size and surface properties of liposomes.
|
| Liposome Pharmacokinetics Study Platform |
- To elucidate the characteristics of liposome drug absorption, distribution, metabolism and excretion in vivo.
- Single/multiple administration pharmacokinetics and dose ratio study.
- Drug sequels and targeting evaluation, etc.
|
| Liposome Biodistribution Study Platform |
- In vivo distribution PK test
- Study on factors affecting the biological distribution of liposomes.
|
| Liposome In Vitro Bioactivity Evaluation Platform |
- Functional activity assay: in vitro cytotoxicity; anticancer activity analysis; anti-inflammatory effects, etc.
- Binding activity assay: evaluation of binding to targeted cells, receptor selective binding analysis.
|
| Liposome Drug Efficacy Evaluation Platform |
- To provide a diverse range of disease assessments.
|
Highlights for Liposome Biological Evaluation
- Diversification. Our liposome biological evaluation services are designed to meet the needs of researchers and scientists working in the fields of pharmaceuticals, biotechnology, and nanotechnology.
- Expertise. Our team of experts has extensive experience in liposome biological evaluations and is committed to staying up-to-date with the latest developments in the field to ensure that our clients receive the most accurate and reliable results, supported by the latest scientific knowledge.
- Cost-effective. The liposome biological evaluation services provided are customizable and cost-effective, tailored to meet the specific needs of individual customers.
CD Formulation has amassed an extensive wealth of expertise and experience in the field of liposome biology, with a focus on understanding their unique distribution patterns in various biological systems. Our seasoned team of researchers and professionals has been at the forefront of this cutting-edge research for many years, consistently striving to unravel the mysteries of liposome behavior. We are well-equipped to address any questions or concerns you may have, and are more than willing to share our knowledge and provide guidance. If you are in need of our assistance, please do not hesitate to contact us at your earliest convenience. We look forward to collaborating with you and supporting your research endeavors.
How It Works
STEP 2
We'll email you to provide your quote and confirm order details if applicable.
STEP 3
Execute the project with real-time communication, and deliver the final report promptly.
Related Services



 Fig.1 Workflow of liposome biological evaluation. (CD Formulation)
Fig.1 Workflow of liposome biological evaluation. (CD Formulation)
