Oral Thin Film Characterization
Inquiry
Oral thin films are considered an alternative to traditional solid and liquid oral dosage forms, which are administered through oral adsorption of the drug, ensuring that the drug goes directly into the systemic circulation, avoiding first-pass effects, acting quickly, more convenient for children and elderly patients who often experience swallowing or nausea problems, and easy to transport and package. CD Formulation is a leader in oral thin film drug delivery, we are committed to providing state-of-the-art services for oral film drug delivery systems to help develop safe and effective oral film drug delivery systems.
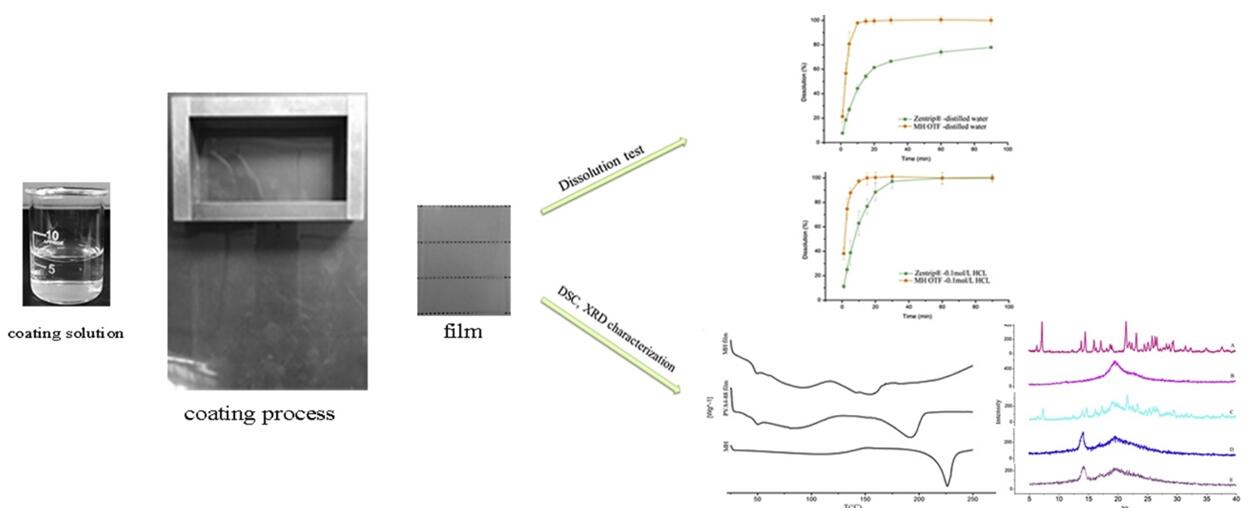 Fig.1 Evaluation of oral thin films in vitro and in vivo. (Yuan Zhao, et al., 2015)
Fig.1 Evaluation of oral thin films in vitro and in vivo. (Yuan Zhao, et al., 2015)
Our Oral Thin Film Characterization Services
Appearance and Physicochemical Properties Characterization
Oral thin films are directly in contact with the oral system and have a high contact area. An enjoyable and palatable drug delivery system is essential to improve patient compliance.
We randomly selected several films with specific surface area and thickness from the prepared oral thin films and dissolved them in distilled water for a certain time to measure the surface pH.
When an orally thin film is in use, its mechanical properties will greatly affect the user experience, including folding endurance, tear resistance, tensile strength, thickness, etc.
Contact angle test of oral thin films to obtain the wettability, surface roughness of films, etc., which can better evaluate the quality of the films.
Test the hygroscopicity of oral thin films under high humidity conditions to control the physical stability and integrity of the films.
The thin films of specific size are precisely weighed and placed on the stencil. The films immersed with the stencil in simulated saliva at a specific temperature. The stencil is removed from the saliva medium at different intervals and the expansion rate is determined by a specific formula.
The transparency of oral thin films is tested using an ultraviolet (UV) spectrophotometer.
Morphology is examined using a SEM. In this way, the presence of smoothness, surface roughness or pores and particle distribution can be determined.
Solid States Analysis
Solid state analysis of oral thin films usually includes XRD/DSC analysis, thermogravimetric (TGA) analysis, fourier transform infrared spectroscopy (FT-IR) analysis, etc., evaluation of solid-state analysis to obtain suitable processing temperature.
Content and Content Uniformity Testing
The oral thin films of specific size are cut from different parts, immersed in the solvent system, dissolved, filtered, and tested.
Moisture Content Testing
The moisture content in the oral thin films will directly affect the quality and efficacy of the drug. If the moisture content is too high, its stability, physical and chemical properties, effectiveness, and validity period will be seriously affected. Therefore, the quantitative analysis of moisture is an important index for the quality control of the oral thin films.
Biological Properties Evaluation
Test the disintegration time of the oral dissolving film in simulated saliva, generally controlled at 20-30s.
In Vitro Dissolution Testing
The biggest obstacle in determining the dissolution rate of the oral thin films is the placement of the films sample. We have made some improvements to the equipment to test the dissolution rate of oral thin films more accurately.
Adhesion Testing
We determine the adhesion of the oral thin films through adhesion strength testing and adhesion time testing to accurately evaluate the effective release time of the drug.
Release Kinetics Testing
The dissolution results of oral thin films in artificial saliva or pure water at pH 6.8 are applied to a computer program to determine an appropriate kinetic model.
What Problems Can We Solve?
Physicochemical and Biological Characterization Services
Relying on the advanced development platform and professional technical teams, we provide physicochemical and biological characterization services of oral thin films, which play a crucial role in the formulation development, optimization of oral thin films and the improvement of drug delivery performance.
Customized Services
We can customize according to the physical and chemical properties of the drug itself, the amount of drug required, and the type of oral film release required, providing multi-angle, personalized and integrated services.
Mechanical and Performance Properties Characterization Services
We perform characterization of the mechanical and performance properties of the films. The mechanical properties include elongation at break, folding resistance and tear resistance. The performance properties include oral thin films uniformity, impurities, and disintegration/dissolution rates.
Our Advantages in Oral Thin Film Characterization
- First-class Expertise. Our researchers have extensive expertise in oral thin film characterization.
- Professional Analysis Services: We provide high-quality oral thin film characterization services and adhere to international quality standards.
- Advanced Oral Thin Film Analysis Platform: We use a wide range of cutting-edge analytical techniques to characterize oral thin films and reveal the complexity of these novel drug delivery systems.
- Efficient Drug Development: Our ability to quickly and accurately characterize oral thin films helps our customers reduce drug development time while maintaining drug accuracy and quality.
CD Formulation is an industry leader in the development and characterization of oral thin films, providing accurate and efficient services for the characterization of oral thin films. If you have a requirement about our oral thin film characterization services, please contact us by phone or email, our colleagues will reply to you within three working days.
References
- Yuan Zhao, Peng Quan, et al. Preparation of an oral thin film containing meclizine hydrochloride: In vitro and in vivo evaluation. International Journal of Pharmaceutics. 2015, Vol (496):314-322.
- Rukiye SEVİNÇ ÖZAKAR, Emrah ÖZAKAR. Current Overview of Oral Thin Films. Turk J Pharm Sci. 2021, 18(1):111-121.
How It Works
STEP 2
We'll email you to provide your quote and confirm order details if applicable.
STEP 3
Execute the project with real-time communication, and deliver the final report promptly.
Related Services

 Fig.1 Evaluation of oral thin films in vitro and in vivo. (Yuan Zhao, et al., 2015)
Fig.1 Evaluation of oral thin films in vitro and in vivo. (Yuan Zhao, et al., 2015)
