Oral Thin Film - CD Formulation
Call Us:
- Home
- Services
- Oral Thin Film Formulation Development
- Quick Release Oral Thin Film Development
- Sublingual Thin Film Development
- Non-Disintegrating Buccal Film Development
- Fast-Disintegrating Buccal Film Development
- Mucoadhesive Sustained-Release Film Development
- 3D Printing Service of Oral Thin Film
- Single-Layer Oral Thin Film Development
- Double-Layer Oral Thin Film Development
- Multi-Layer Oral Thin Film Development
- One / Several APIs Oral Thin Film Development
- Novel Solvent-free Oral Thin Film Development
- Oral Thin Film Process Development
- APIs Screening and Characterization of Oral Thin Films
- Excipient Ingredient Evaluation of Oral Thin Film
- Preparation Method Screening of Oral Thin Films
- Solvent Casting Method for Oral Thin Film Preparation
- Hot Melt Extrusion Method for Oral Thin Film Preparation
- Semi-solid Casting Method for Oral Thin Film Preparation
- Solid Dispersion Extrusion Method for Oral Thin Film Preparation
- Rolling Method for Oral Thin Film Preparation
- 3D Printing Method for Oral Thin Film Preparation
- Oral Thin Film Characterization
- Oral Thin Film Organoleptic Evaluation
- Oral Thin Film Surface pH Testing
- Oral Thin Film Mechanical Properties Characterization
- Oral Thin Film Contact Angle Measurement
- Oral Thin Film Moisture Absorption Testing
- Oral Thin Film Swelling Properties Testing
- Oral Thin Film Transparency Testing
- Oral Thin Film Morphology Testing
- Oral Thin Film Content Testing
- Oral Thin Film Solid State Analysis
- Oral Thin Film Content Uniformity Testing
- Oral Thin Film Moisture Content Testing
- Oral Thin Film In Vitro Disintegration Testing
- Oral Thin Film In Vitro Dissolution Testing
- Oral Thin Film Adhesion Testing
- Oral Thin Film Irritation Study
- Oral Thin Film Release Kinetics Testing
- Oral Thin Film Efficacy Evaluation
- Oral Thin Film Analysis and Testing Services
- Oral Thin Film Manufacturing and Packaging Services
- Oral Thin Film Formulation Development
- Technologies
- Oral Thin Film Quality & Analytical Technologies
- Solubilization Technologies for Poorly Soluble Drugs
- Oral Thin Film Manufacturing Technologies
- Solvent Casting Technology for Oral Thin Film Manufacturing
- Hot Melt Extrusion Technology for Oral Thin Film Manufacturing
- Semi-solid Casting Technology for Oral Thin Film Manufacturing
- Solid Dispersion Extrusion Technology for Oral Thin Film Manufacturing
- Rolling Technology for Oral Thin Film Manufacturing
- 3D Printing Technology for Oral Thin Film Manufacturing
- Oral Thin Film Biologics Delivery Technologies
- Applications
- Oral Thin Film for Medical Areas
- Oral Thin Film for Cardiovascular Therapeutic Research
- Oral Thin Film for Pain Relief Research
- Oral Thin Film for Mood or Mental Disorder Therapy Research
- Oral Thin Film for Respiratory Disease Therapy Research
- Antihistamines Oral Thin Film Formulation Research
- Oral Thin Film for Erectile Dysfunction Therapy Research
- Oral Thin Film for Health Products
- Oral Thin Film for Food Industry
- Oral Thin Film for Cosmetic Industry
- Oral Thin Film for Medical Areas
- Order Online
- Company
- Inquiry


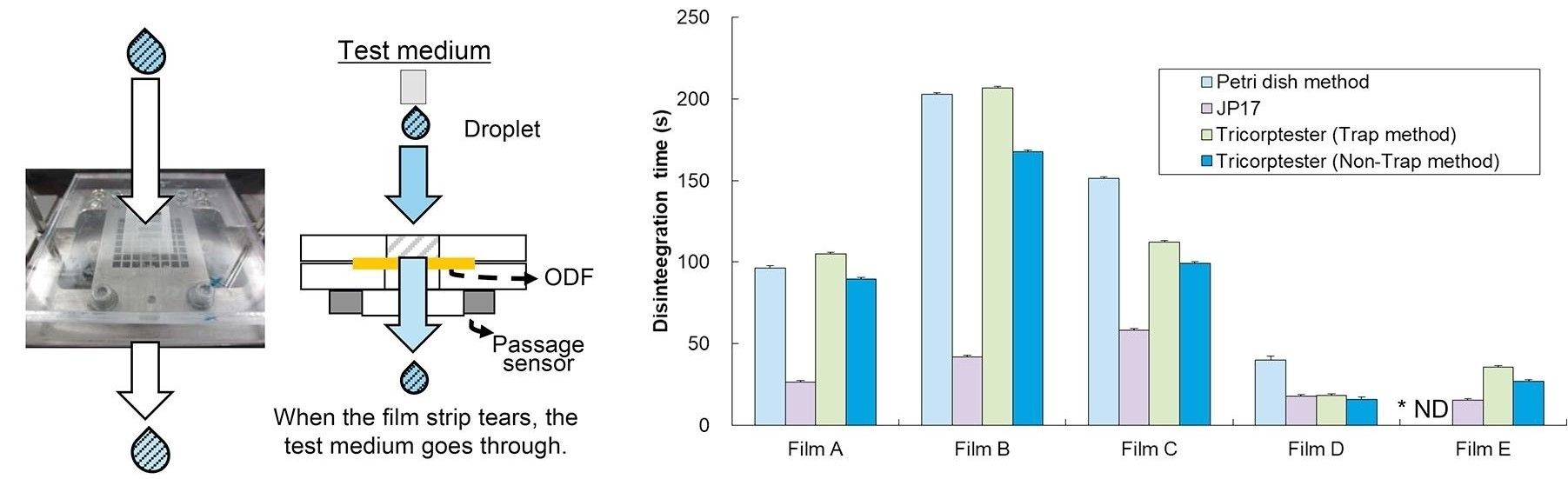 Fig.1 A new disintegration test system of oral films. (Yoshiko Takeuchi, et al., 2018)
Fig.1 A new disintegration test system of oral films. (Yoshiko Takeuchi, et al., 2018) Fig.2 The Importance of Oral Thin Film In Vitro Disintegration Testing. (CD Formulation)
Fig.2 The Importance of Oral Thin Film In Vitro Disintegration Testing. (CD Formulation)
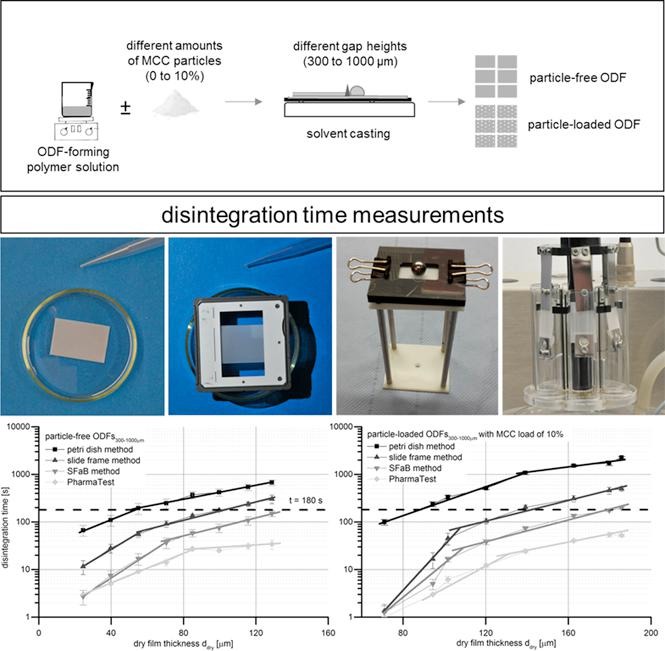 Fig.3 Comparative study on disintegration methods for oral film preparations. (Isabell Speer, et al., 2018)
Fig.3 Comparative study on disintegration methods for oral film preparations. (Isabell Speer, et al., 2018)
