Viral Gene Delivery Systems Development
Inquiry
Gene delivery systems that rely on viral vectors can meet key criteria for efficient in vivo delivery of vectors, while viruses have evolved to overcome barriers to in vivo delivery and can naturally deliver nucleic acid vectors to many cell types, and based on these favorable attributes, viruses have emerged as important vectors for the delivery of gene therapy agents.
CD Formulation provides professional and reliable technical support services for the development of viral gene delivery systems by relying on its advanced technology platform and experienced team of experts.
Advantages of Viral Gene Delivery Systems Development
- Efficient gene transfection. Relying on viral vectors, it is able to efficiently enter host cells and release their genomes, thus providing efficient gene transfection.
- Specific targeting. Designing viruses to target specific types of cells can greatly improve the effectiveness and targeting of gene therapy formulations and reduce side effects.
- Sustained expression. Some viruses, such as adenoviruses and lentiviruses, are capable of sustained expression of exogenous genes within the cell, providing long-lasting expression when applied to drug delivery of gene therapy agents.
- Large vector capacity. Viral vectors are able to carry larger-sized genomes and have a larger vector capacity than other gene delivery systems, such as plasmid-based gene delivery systems.
Explore Our Viral Gene Delivery Systems Development
We are committed to providing comprehensive and reliable viral gene delivery system development services to support the development and optimization of gene therapy formulations. Our professional team has extensive experience in customizing and developing efficient gene delivery solutions based on the specific needs of our clients. Below are the services we currently offer.
We offer AAV vector development services designed to meet the complex needs of researchers, including improving manufacturing processes and optimizing delivery mechanisms to create high-quality, reliable AAV vectors. This service we provide is critical in helping researchers advance the development of gene therapy agents and accelerate the progress of their program studies. Not only can we help researchers gain expertise and streamline the project process, but we can also help them fully utilize the potential of AAV vectors.
Adenoviruses, which enter cells through receptor-mediated endocytosis and then transfer the adenoviral genome to the nucleus for infection of dividing and non-dividing cells, have an important role in the development of gene therapy agents. Our extensive service experience in the development of adenoviral vectors has greatly contributed to the progress of adenoviral vector development and application.
Lentiviral vectors integrate exogenous genes into the host genome by infecting dividing and non-dividing cells for long-term stable expression. We provide comprehensive lentiviral vector development services, including the entire process from vector design to production.
Retroviral vector development is widely used as a gene delivery vector for the development of gene therapy agents. We provide retroviral services from vector design to quality control to facilitate the development of gene therapy agents.
Herpes simplex virus vectors are now widely used in gene therapy formulation development studies for neurological diseases due to their ability to effectively infect nerve cells and their large gene vector capacity. We provide innovative and reliable herpes simplex virus vector development services according to researchers' development needs, aiming to improve safe and effective gene delivery programs.
Vaccinia viral is a large and complex enveloped virus that is widely used for high-level cytoplasmic transgene expression and is currently widely used as a viral vector for gene therapy due to its large genome, high transduction efficiency, and ability to carry large amounts of exogenous genes. We offer highly attenuated, replicative Vaccinia viral vector development services to meet gene delivery needs.
Baculovirus can introduce exogenous genes into host cells and express target proteins with high efficiency, which is characterized by a wide range of applications and high application potential, especially in gene therapy agent development. We provide a full range of Baculovirus vector development services, providing an effective solution for gene drug delivery.
Our Process of Viral Gene Delivery Systems Development
- Target gene selection and design. Identification of target genes and design of transgenes capable of repairing or replacing defective genes.
- Selection and construction of viral vectors. Select appropriate viral vectors according to the size of the target gene and therapeutic needs, and insert the target gene into the viral vector to ensure that the viral vector can carry and deliver the target gene.
- Packaging and purification of viral vector. The constructed viral vector DNA is co-transfected with the viral capsid protein into the host cell, so that the viral vector can replicate itself and assemble into mature viral particles in the host cell, then the viral particles are collected and impurities are removed through the purification step.
- Viral vector evaluation and optimization. Evaluate the transduction efficiency and expression level of viral vectors, as well as the safety and efficacy of viral vectors. Based on the evaluation results, the viral vectors are optimized.
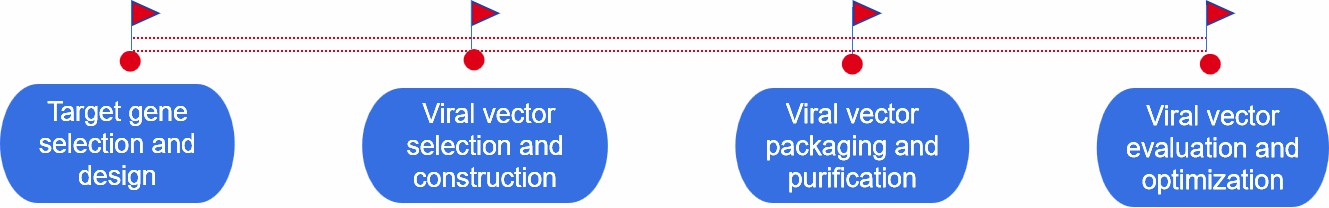 Fig.1 Our development process of viral gene delivery systems. (CD Formulation)
Fig.1 Our development process of viral gene delivery systems. (CD Formulation)
Our Platforms for Viral Gene Delivery Systems Development
| Technologies & Platforms |
Content Description |
| Chromatography-based purification systems |
Purification is a critical step in producing high-quality viral vectors free from contaminants. Chromatography platforms are the standard for purifying viral particles to meet higher-grade requirements. |
| CRISPR/Cas9 technology |
CRISPR/Cas9 can be used to select, design, and modify target genes. It can also be used to construct viral vectors to insert or delete specific sequences through precise gene editing. Based on gene editing as a technology platform, we can help researchers customize therapeutic genes and integrate them into viral vectors. |
| Next generation sequencing |
NGS is critical in the selection and design of target genes. It helps researchers analyze genetic variation, identify mutations and understand gene expression profiles in an efficient and comprehensive manner, facilitating the construction and confirmation of target genes. |
Highlights of Our Viral Gene Delivery Systems Development Service
- We offer custom development services to fully meet the specific needs of researchers in the development of viral gene delivery systems.
- Our professional team has rich experience in gene therapy development and strictly controls every step of the process to ensure high-quality vector design and purification.
- Our one-stop development service covers all aspects of viral gene delivery system development, which greatly enhances project research efficiency.
- With our advanced technology platform and innovative solutions, we continuously optimize viral vector design to ensure high purity, stability and expression efficiency.
Published Data
Technology: Viral gene delivery technology
Journal: Adv Drug Deliv Rev
IF: 16.1
Published: 2006
Results: Genetic modification is achieved through gene delivery, either using viral transduction or non-viral transfection systems and although novel non-viral systems continue to emerge as innovative vectors for the control of gene delivery, viruses remain the most effective means of introducing exogenous genes into mammalian cells and their expression by them. This article discusses the genome structure of various viral types, including retroviruses, adenoviruses, and adeno-associated viruses, as well as their advantages and disadvantages for tissue engineering applications.
CD Formulation is an industry leader in the development of viral gene delivery systems and has been recognized by customers and industry insiders alike. If you are interested in us, please feel free to contact us.
References
- Zhang X, Godbey WT. Viral vectors for gene delivery in tissue engineering. Adv Drug Deliv Rev. 2006, 58(4):515-34.
Related Services


 Fig.1 Our development process of viral gene delivery systems. (CD Formulation)
Fig.1 Our development process of viral gene delivery systems. (CD Formulation)