Herpes Simplex Virus Vector Development Service
Inquiry
Herpes simplex virus (HSV) is a common human pathogen, and humans are its natural hosts. Structurally, HSV viruses are spherical, with multilayered concentric structures. Because HSV can establish a latent infection in these cells, it is particularly well suited to deliver genes to neurons. Advances in molecular biology and viral genetics have made possible the use of viral vectors for gene therapy, whereby exogenous genes are packaged into such vectors that can be targeted to specific regions of the nervous system for the treatment of neurological disorders such as malignant gliomas, Parkinson's disease, known monogenic disorders, and cerebral ischemia. CD Formulation provides high-quality HSV vector development services for gene therapy delivery system development.
Advantages of HSV Vector Development
Recombinant HSV is an ideal viral vector for in vivo and in vitro gene transduction experiments in gene therapy research, and has multiple application advantages.
- Natural neurophilicity. After replication in epithelial cells, HSV produces progeny virus particles that can further infect sensory neurons and then pass along axons into neuronal cell bodies.
- Long-term latency. HSV-1 can be latently expressed in neuronal cells through the latency-associated transcriptional region (LAT). HSV-1 vectors have become the most effective gene expression vectors in the nervous system, and have unique advantages in the treatment of neurological diseases.
- Large vector capacity. In terms of loading capacity, HSV-1 can carry about 30kb of exogenous genes, which is 8-fold higher than that of the commonly used AAV vectors (AAV vectors can carry about 4kb of exogenous genes). Based on the higher loading capacity of the HSV-1 vector, it can carry multiple therapeutic genes, which can address the shortcomings of AAV vectors in loading larger genes or in the treatment of multi-gene diseases.
- Wide host cell spectrum. HSV-1 vectors have a wide host cell spectrum, and the modified replication-defective vectors can efficiently deliver target genes to a wide range of cell types, which applies to the treatment of various diseases.
What We Offer to Develop HSV Vectors?
Gene selection
Selection of suitable genes as therapeutic targets, genes with important roles in herpes simplex virus type I infection, and disease progression are ideal for selection. The gene should have potential therapeutic effects and be modifiable.
Vector design
Designing an HSV vector suitable for gene therapy requires knocking out certain essential genes of the virus to ensure that the virus is unable to replicate in normal cells while maintaining its ability to replicate in the target cells.
Genome editing
Modern gene editing techniques such as CRISPR-Cas9 are utilized to make precise modifications to the HSV genome, including the insertion of therapeutic genes and the removal of certain genes from the virus to reduce its virulence.
Viral vector construction
Recombinant HSV vectors are constructed by homologous recombination or bacterial artificial chromosome (BAC) technology, which allows manipulation of the viral genome in E. coli, while yeast artificial chromosome (YAC) technology supports replication and modification of the genome in yeast.
Virus production
Viral vectors are produced in engineered cell lines. These cell lines are designed to support viral replication and packaging while expressing desired therapeutic genes.
Virus purification
Produced viruses undergo a purification step to ensure the quality and safety of the viral vector. This may include purification of the viral pellet using techniques such as sucrose density gradient centrifugation.
Applications of Our HSV Vector Development Service
Gene delivery using HSV vectors is a frontier area for gene therapy applications. Recombinant HSV has a wide range of applications in neurological gene delivery, oncolytic gene transduction, and vaccine development.
- Applications in tumor research. HSV-1 is highly infectious and can rapidly and efficiently spread within solid tumors. A specially designed attenuated HSV oncolytic virus can selectively replicate in tumor cells and kill them. In addition, the tumor-killing effect of this HSV can be further enhanced by carrying anticancer genes or genes that activate chemotherapeutic reagents.
- Applications in vaccines. Attenuated HSVs are particularly suitable for the development of anti-HSV vaccines because they provide the host's immune system with exposure to multiple types of viral antigens, which can activate cellular and humoral immunity.
- Applications in neurological gene delivery. HSV amplicon vectors are based on HSV-1 but contain only one or more target genes and minimal viral genome sequences HSV amplicon vectors are particularly suitable for gene delivery to neurons due to their natural neurophilicity, great loading capacity, low toxicity, and the presence of the viral genome sequences as a free body within the target cell.
Our Platforms for HSV Vectors Development
| Technologies & Platforms |
Content Description |
| Gene synthetic technology platform |
The target viral vectors constructed by the gene synthesis technique exhibited high infectivity as well as excellent replication ability at the cellular level. This technology has played a crucial role in the development of herpesvirus vectors, and we have been able to utilize this technology to help scientists edit and improve viral vectors with greater precision, thereby advancing the development and application of viral vectors in gene therapy. |
| Viral vector modification technology platform |
Using gene editing technology, we can modify HSV to improve its efficiency and safety as a vector. For example, with the CRISPR-Cas9 system, recombinant HSV-1 vectors can be rapidly constructed for oncolytic virus therapy and gene transfer in tumors. |
| DNA recombination technology platform |
Recombinant gene technology has made it possible to construct recombinant HSV vectors that can be used for gene therapy, vaccine development, and lysosomal virus therapy. For example, by inserting therapeutic genes or immunostimulatory factors (e.g., hGM-CSF expression cassettes) into HSV vectors, recombinant viruses with therapeutic potential for treating tumors can be developed. |
Highlights of Our HSV Vector Development Service
- Comprehensive support. We provide a full range of technical support and consulting services to help you solve problems encountered during HSV vector construction and application.
- Reliable quality. The HSV vectors we developed are characterized by high titer, high purity, low endotoxin and high in vivo infectivity.
- Experienced. With years of experience in viral vector development services, we can help researchers rapidly advance HSV vectors in gene therapy.
Published Data
Technology: Gene synthetic technology
Journal: Virol Sin
IF: 3.2
Published: 2023
Results: HSV-1 H129 is a clinical isolate of herpes simplex virus type I with unique cis-trans-synaptic transmission properties, which has been widely used as a vector in the field of neural loop tracing. However, it is difficult to edit HSV-1 at the genome-wide level by conventional molecular biology methods, which limits the further application of HSV-1 virus. Using the neural tracer virus H129-G4 as a template, the team synthesized the whole genome of the virus with a green fluorescent gene in yeast using synthetic technology, and transfected it into mammalian cells, successfully rescuing H129-Syn-G2. The study demonstrated that the virus has similar infectivity and morphological characteristics as the parental virus, and its cellular level replication ability is better than that of the template virus.
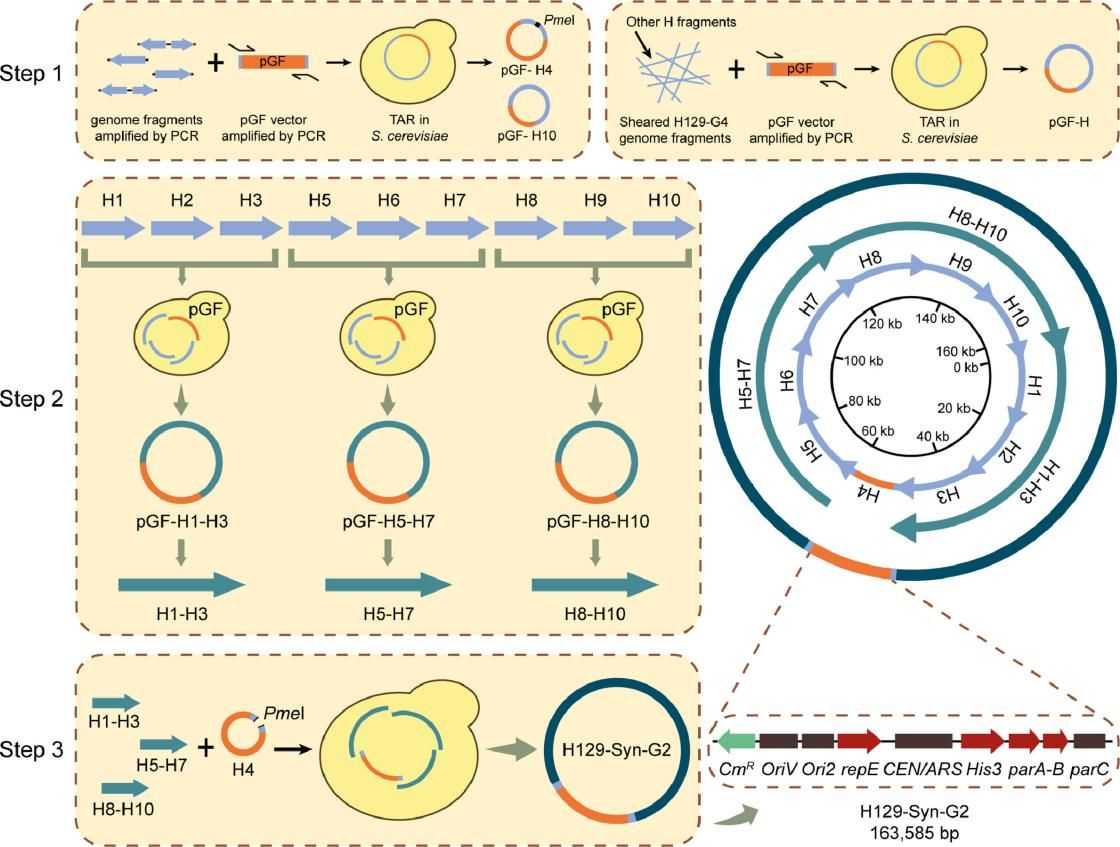 Fig.1 Schematic flowchart of the synthesis of H129-Syn-G2. (Xiao H, et al., 2023)
Fig.1 Schematic flowchart of the synthesis of H129-Syn-G2. (Xiao H, et al., 2023)
CD Formulation as a global leader in scientific services, we provide innovative solutions to accelerate the development of HSV vectors to help advance the field of gene therapy. If you are interested in us, please feel free to contact us.
Reference
- Xiao H, Hu H, et al. Construction and characterization of a synthesized herpes simplex virus H129-Syn-G2. Virol Sin. 2023 , 38(3):373-379.
Related Services

 Fig.1 Schematic flowchart of the synthesis of H129-Syn-G2. (Xiao H, et al., 2023)
Fig.1 Schematic flowchart of the synthesis of H129-Syn-G2. (Xiao H, et al., 2023)