Lentiviral Vector Development Service
Inquiry
Lentivirus vector are gene therapy vectors developed on the basis of HIV-1 (Human Immunodeficiency Virus Type I). Lentivirus vector is commonly used in cell and gene therapy. It is capable of infecting both dividing and non-dividing cells, and can use reverse transcriptase to integrate exogenous genes into the host genome and express them stably over a long period of time, e.g., in neurons, hematopoietic stem cells and cells of the immune system. CD Formulation provides comprehensive lentiviral vectors with multiple cutting-edge modifications used for transgene delivery studies and application.
Advantages of Lentiviral Vectors Development
Lentiviral vectors have many advantages that make them an effective tool for the efficient introduction of exogenous genes and the application of gene therapy for diseases and have good application prospects.
- High gene transduction efficiency. Compared with other retroviruses, lentiviruses have a wider range of hosts and are capable of infecting both dividing and non-dividing cells. For some difficult-to-transfect cells, such as primary cells, stem cells, and undifferentiated cells, lentiviral vectors can greatly improve the efficiency of target gene transduction, and greatly increase the chances of integrating the target genes into the genome of the host cells.
- Efficient and stable expression. The target genes carried by lentiviral vectors and integrated into host cells are resistant to transcriptional silencing and can be expressed in target cells with high efficiency and stability.
- Expression in specific tissues. Lentiviral vectors can be inserted with tissue cell-specific promoters and enhancers to improve the transferred genes' transcriptional targeting so that the lentiviral vectors' target genes can be expressed in specific tissue cells.
- Carrying longer target genes. The constructed lentiviral vectors can carry target genes of about 5 kb or even longer so that many cDNAs can be cloned into lentiviral vectors in addition to small molecules such as exogenous short-hairpin RNAs (shRNAs).
What We Offer to Develop Lentiviral Vectors?
Selection of target gene
We first need to identify the target gene for research or treatment, i.e. the transcript of the target gene.
Selection of vector backbone
Select the appropriate lentiviral vector backbone and corresponding components according to the experimental needs.
Optimization of components
Lentiviral vectors contain several components, such as RRE, CPPT, Kozak, WPRE, T2a, etc. These components serve to improve the lentiviral packaging efficiency, titer, and expression efficiency of the target gene, etc.
Acquisition of target gene fragments
Target fragments with enzymatic sites can be obtained by direct synthesis or PCR amplification.
Vector construction
The target fragment is ligated into the lentiviral vector, and the recombinant vector is formed by connecting the target fragment to the vector backbone through enzymatic cleavage and ligase.
Cell transfection
Transform the recombinant vector into receptor cells amplify the recombinant vector, and then confirm the accuracy by sequencing.
The purified and concentrated viral vector is obtained
Finally, we harvest the viral supernatant from the expanded culture and purify and concentrate it to obtain specific lentiviral vectors.
Numerous clients have acknowledged the special benefits of our lentiviral vector development for gene transfection. Being at the forefront of lentiviral vector development means that we are consistently developing new lentiviral systems or improving existing ones to make them more flexible for a variety of transgenic applications. Furthermore, we persist in removing technical obstacles in order to enhance virus titers even further.
Furthermore, we provide an array of targeting optimization techniques for lentiviral vectors, such as direct targeting, selective targeting to particular tissues, tissue-specific promoter-based transgene expression control, and miRNA-mediated silencing. Researchers are welcome to contact us.
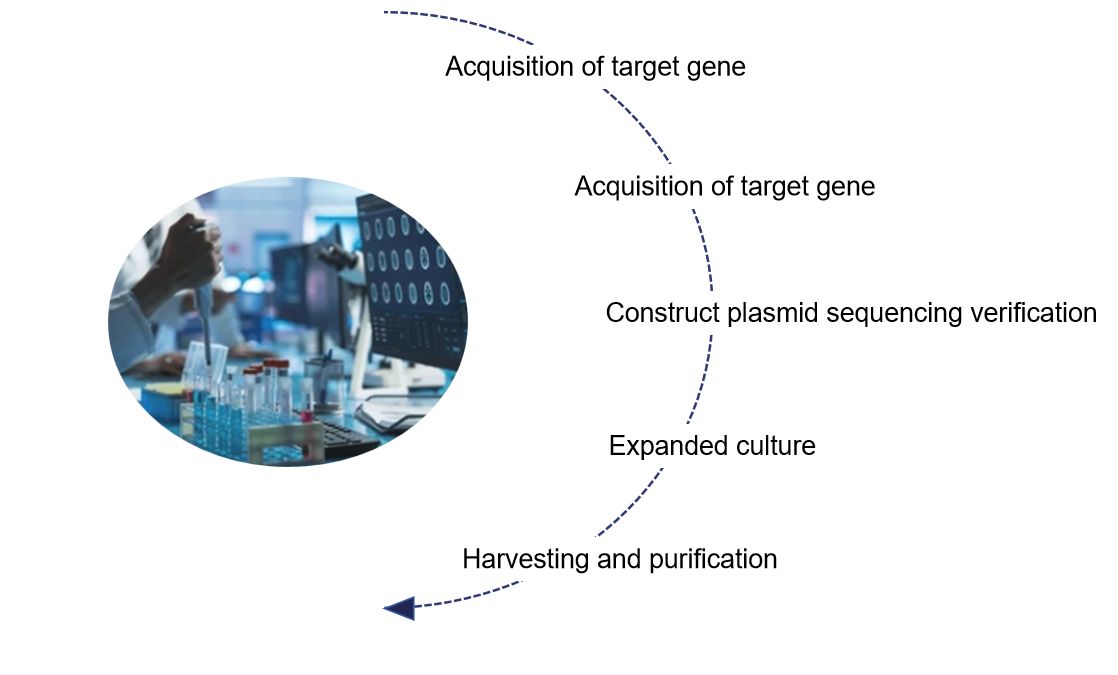 Fig.1 Our development process of lentiviral vectors. (CD Formulation)
Fig.1 Our development process of lentiviral vectors. (CD Formulation)
Application of Lentiviral Vector Development
- Application in tumor treatment. The occurrence of cancer is mainly related to gene mutation. As a new method, gene therapy mainly introduces the target gene into the target cell. For abnormal genes in the process of tumor occurrence and development, gene fragments with therapeutic value are introduced, and they are effectively and permanently expressed in the target cells to achieve the purpose of treatment. Some biological characteristics of lentivirus make the idea of gene therapy possible. If lentivirus is used in the clinical treatment of tumor diseases, it may bring new progress to the treatment of diseases. At present, the development of lentivirus vectors has been widely used in basic research on malignant tumors such as osteosarcoma, leukemia, liver cancer, and nasopharyngeal carcinoma.
- Application in nervous system diseases. Lentiviral vectors can stably transfect most non-dividing primary cells, including neurons, and then can be expressed stably and continuously, playing a therapeutic role. A large number of studies have shown that the use of lentiviral vectors to express genes related to various nervous system diseases has achieved good results in long-term treatment in animal models of Parkinson's disease, Alzheimer's disease, spinal cord injury and other diseases.
Our Platforms for Adenovirus Vector Development
| Technologies & Platforms |
Content Description |
| LV-RNAi technology platform |
LV-RNAi has a long-lasting effect, and the range of cells infected by the vector is expanded. It can be used for the study of specific gene functions of cells and for gene therapy. The combination of the advantages of the two technologies has become a new method for the treatment of tumor diseases. LV can safely transfer tumor therapeutic genes into target cells and is widely used in basic research on a variety of tumors. |
| Gene synthesis technology platform |
We offer custom gene synthesis as a reliable and cost-effective method to provide customers with custom DNA constructs with 100% sequence accuracy. |
| Suspension serum-free production technology platform |
The viruses produced by this technology platform provided by us have the advantages of high supernatant viral titer, good viral activity, low impurity residue, no protein content in the raw material for production, easy to produce and scale up, simple production process, and low cost. |
| Virus-likeparticles (VLP) delivery technology |
VLP refers to protein particles formed by the self-assembly of single or multiple structural proteins of viruses, such as capsid proteins and coat proteins, which are highly similar to the structure of natural viral particles, do not have viral genetic material, and are easy to be infected and enter into the cells, and can realize the intracellular delivery of proteins and genetic material, and is a very promising gene delivery vector for clinical use. |
Highlights of Our Lentiviral Vector Development Services
- Professional team. We have a professional research team, with profound professional knowledge and technical strength, we can provide you with customized lentiviral vector construction solutions.
- Customized design. We will customize the most suitable lentiviral vector construction solution for you according to your research needs.
- Diverse vector types. We are able to construct multiple types of lentiviral vectors, including integrated and non-integrated lentiviral vectors.
- AI artificial intelligence plus. We have a strong AI and BioTrust team that can provide lentiviral vector development and optimization and transformation services.
Published Data
Technology: Gene synthesis technology platform
Journal: Blood.
IF: 22
Published: 2000
Results: In this study, the authors explore the development of lentiviral vectors, particularly for HIV-1 in detail, highlighting the advantages of lentiviruses over oncogenic retroviruses in infecting non-dividing and terminally differentiated cells. It was also found that the infectivity, stability, and targeting of the vectors could be significantly improved by improved lentiviral vector design, such as the use of VSV-G envelope glycoproteins and optimized internal promoters.
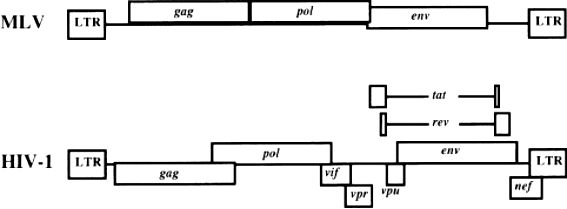 Fig.2 Genomes of lentivirus. (Buchschacher GL, et al., 2000)
Fig.2 Genomes of lentivirus. (Buchschacher GL, et al., 2000)
CD Formulation provides lentiviral vector development services using an industry-recognized standard process that combines the advantages of second- and third-generation lentiviral systems to ensure both biosafety and high titer viruses. The lentiviral vectors constructed by us undergo strict quality testing and can greatly meet the research needs of our clients' openings.If you are interested in us, please feel free to contact us.
Reference
- Buchschacher GL Jr, Wong-Staal F. Development of lentiviral vectors for gene therapy for human diseases. Blood. 2000, 95(8):2499-504.
Related Services

 Fig.1 Our development process of lentiviral vectors. (CD Formulation)
Fig.1 Our development process of lentiviral vectors. (CD Formulation) Fig.2 Genomes of lentivirus. (Buchschacher GL, et al., 2000)
Fig.2 Genomes of lentivirus. (Buchschacher GL, et al., 2000)