Baculovirus Vector Development Service
Inquiry
Baculovirus expression vectors utilize baculoviruses as the basis for introducing exogenous genes into host cells for efficient expression of target proteins. With the continuous development of genetic engineering technology, baculoviral expression vectors have been expanding their applications and have shown great potential in gene therapy vaccine development, etc. CD Formulation is a leading global biotechnology service provider with an experienced research team and multiple advanced technology platforms to provide a full range of baculoviral vector development services, such as We offer a full range of baculovirus vector development services, such as baculovirus transfer vector construction, recombinant baculovirus DNA preparation, baculovirus production and baculovirus titer assay.
Advantages of Baculovirus Vector Development
- Efficient delivery of exogenous genes. Bacteriophages have high infectivity in nature and can effectively invade host cells and integrate their genomes into the host genome. Taking advantage of this property, researchers can embed exogenous genes of interest into the genome of baculoviruses, and introduce them into the target cells through appropriate transfection or infection methods, thus realizing the expression of the target genes.
- Wide range of host cells. The ability of baculoviruses to infect a wide range of cell types, including mammalian cells, plant cells, etc., allows them to be applied in different biological systems. This provides researchers with the convenience of conducting gene expression studies in different experimental models and is expected to be useful in clinical applications such as gene therapy.
- Higher safety and stability. Modified baculovirus vectors are usually able to stably exist and express target genes in host cells while having less impact on host cells and not causing severe immune responses or cytotoxicity.
What We Offer to Develop Baculovirus Vectors?
With the advancement of biotechnology, the design and construction of baculovirus expression vectors are constantly improving. Our researchers are developing new vector construction strategies to further improve the expression efficiency and stability of vectors by altering the genomic structure of vectors or introducing specific genomic modifications to meet the needs of different studies and applications. The development of our baculovirus vectors for application in gene therapy involves several key processes.
Vector construction
In this process, we need to select baculoviruses suitable for use as gene therapy vectors and extract their genomes. Subsequently, we modify the vector, including deleting or eliminating disease-causing genes from the baculovirus genome, inserting sequences carrying therapeutic genes, and enhancing the specificity of the virus for the target cells.
Gene insertion
We select the therapeutic genes to be delivered by selecting the therapeutic genes in half, such as repair genes and anticancer genes. Subsequently, we utilize our advanced molecular technology platform to construct therapeutic gene expression vectors, i.e., the constructed therapeutic gene fragments are inserted into specific sites in the genome of the baculovirus vector.
Vector amplification
To produce an adequate amount of viral particles, we amplify the modified baculovirus vectors in a bioreactor or cell culture system. To produce high-quality viral vectors, the viral particles and viral products are additionally purified and concentrated.
In vitro and in vivo assays
An in vivo viral titer assay is used in conjunction with the in vitro assay to ascertain the efficient dosage of delivery by measuring the virus's titer. Furthermore, we must carry out quality control on the generated viral particles, examining their genetic stability, activity, and purity. A set of animal and cellular testing is called an in vivo assay.
 Fig.1 Our development process of baculovirus vectors. (CD Formulation)
Fig.1 Our development process of baculovirus vectors. (CD Formulation)
Applications of Our Baculovirus Vector Development Service
Our extensive knowledge of baculovirus-based development will facilitate the design of improved second-generation viruses for gene transfer. We can construct simplicity and acceptance of exogenous large fragment DNA (>20kb) baculoviruses that have enhanced or targeted hydrophilicity, as well as more stable transgene expression and control over the timing and cell type specificity of transgene expression. The baculovirus vectors we have developed are capable of being used in gene therapy studies for a variety of diseases, including liver cancer, hemophilia, muscular dystrophy, and retinal diseases. Below we focus on applications in retinal diseases and tumors.
- Treatment of hereditary retinopathy. Rod-shaped viral vectors have been used to deliver retinal disease-related genes, and have been successful in treating diseases such as congenital melanocytic epithelial dystrophy and retinitis pigmentosa. The vectors were developed to deliver normal replicating genes to retinal cells to restore visual function. In addition, baculovirus vectors can be used to deliver neuroprotective and repair factors to promote the survival and recovery of damaged retinal cells. This gene therapy may improve vision by promoting regeneration and reconstruction of nerve cells after retinal damage.
- Treatment of certain tumors. Sapoviral vectors can be used to deliver anti-tumor genes that induce apoptosis or inhibit the growth of tumor cells. For example, anti-tumor drug sensitivity genes can be delivered to cancer cells to sensitize them to chemotherapeutic drugs, thereby enhancing therapeutic efficacy. In addition, the delivery of an immunostimulatory gene via a baculovirus vector can enhance the ability of the immune system to recognize and attack tumors. This immunotherapy activates the killing ability of T cells and improves the immune recognition of tumor cells.
Our Platforms for Baculovirus Vector Development
| Technologies & Platforms |
Content Description |
| Baculovirus expression vector system |
BEVS is a well-established exogenous protein expression platform for the production of biologically active recombinant products, including recombinant proteins for vaccines. BEVS consists of a transfer plasmid, a baculovirus vector, and an insect host cell line. Commonly used baculoviral vectors include AcMNPV and BmNPV, and host cell lines such as Sf9 and Hi5 are used for viral amplification and protein expression. |
| Vector optimization technology platform |
Enhance the expression level of recombinant proteins by regulating DNA elements in regions near the target gene. For example, optimizing vector elements such as promoters and enhancers to enhance protein expression. |
| Meganuclease technology platform |
This technology platform allows for the recognition of sequence-specific DNA cleaving enzymes or nucleic acid endonucleases for specific larger (12 to 40 bp) DNA sequences. |
| Molecular biology technology platform |
We utilize genetic engineering techniques to modify the baculovirus genome to insert therapeutic genes. Among them, gene editing techniques such as CRISPR-Cas9 can be used to perform precise gene insertion, replacement or targeted modification in baculovirus vectors for the expression or silencing of specific genes. This technology can improve the precision and efficiency of gene delivery in vectors. |
Highlights of Our Baculovirus Vector Development Service
- Advanced technology platform. We have advanced molecular biology and genetic engineering technology platforms to ensure that the design, construction, and optimization of baculovirus vectors are at the leading level in the industry.
- Innovative solutions. Our researchers are constantly developing new vector construction strategies to further improve the expression efficiency and stability of vectors by altering the genomic structure of vectors or introducing specific genomic modifications, providing innovative solutions for our customers.
- One-stop services. We provide a one-stop solution for the development of baculovirus vectors for gene therapy, which can help our customers control time and cost to a great extent.
- Strict quality control. We employ stringent quality control procedures to ensure that the baculovirus vectors we provide have high purity, high stability, and high expression efficiency.
Published Data
Technology: Molecular biology technology platform
Journal: Mol Biotechnol
IF: 2.6
Published: 2010
Results: In this study, the insect cell-specific baculovirus Autographa californica multiple nucleopolyhedrovirus (AcMNPV) has been evaluated as a vector for gene delivery to colorectal cancer cells. Experiments involving factorial design were employed to study the individual and combined effects of different parameters such as multiplicity of infection (MOI), viral incubation time, and epigenetic factors on transduction efficiency. The results demonstrate that baculovirus gene delivery system holds immense potential for the development of a new generation of highly effective virotherapy for colorectal, as well as other major carcinomas (breast, pancreas, and brain), and offers significant benefits to traditional animal virus-based vectors concerning safety concerns.
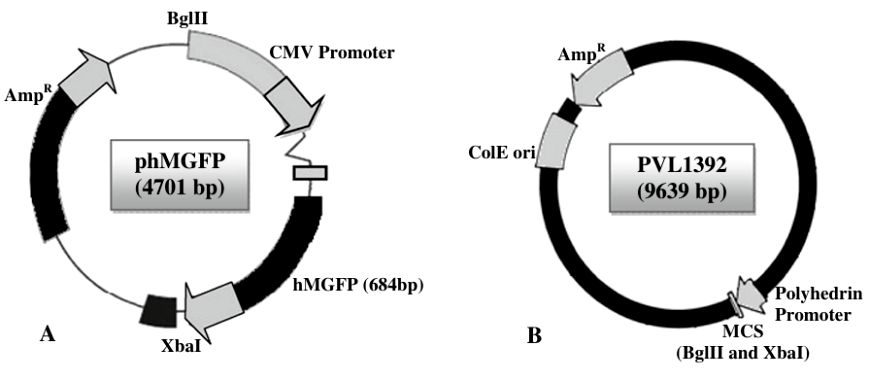 Fig.2 Cloning of recombinant baculovirus vectors. (Paul A, et al., 2010)
Fig.2 Cloning of recombinant baculovirus vectors. (Paul A, et al., 2010)
CD Formulation has state-of-the-art facilities and experienced staff to assist in all areas of viral vector design and construction, and we can provide high-quality, full-process baculovirus development services based on the needs of the investigator's project, ensuring the advancement of baculovirus delivery systems for research and application in gene therapy. As well as rapid virus generation. We provide a one-stop service for your project, including the design and construction of suitable vectors for gene delivery. If you are interested in us, please feel free to contact us.
Reference
- Paul A, Jardin BA, et al. Recombinant baculovirus as a highly potent vector for gene therapy of human colorectal carcinoma: molecular cloning, expression, and in vitro characterization. Mol Biotechnol. 2010, 45(2):129-39.
Related Services

 Fig.1 Our development process of baculovirus vectors. (CD Formulation)
Fig.1 Our development process of baculovirus vectors. (CD Formulation) Fig.2 Cloning of recombinant baculovirus vectors. (Paul A, et al., 2010)
Fig.2 Cloning of recombinant baculovirus vectors. (Paul A, et al., 2010)