Adeno-Associated Viral Vector Development Service
Inquiry
Adeno-associated virus (AAV) vectors are pivotal in the field of gene therapy and are one of the candidate gene therapy vectors due to their low infectivity, low immune response, and ability to transduce multiple tissues. Utilizing the fundamentals of molecular biology and laboratory techniques, CD Formulation offers a wide range of AAV vector development services for basic research.
Advantages of AAV Vector Development
- High safety. Wild-type AAV has never been found to be pathogenic to humans, and the recombinant AAV genome sequence removes most of the wild-type AAV genome elements, which further ensures safety.
- Low immunogenicity. When AAV infects muscle, brain, eye, and other tissues with localized large doses, few infected cells are cleared by the immune system afterward, which is extremely helpful for animal experiments.
- Wide host range. Almost all cells in the division and quiescent phase can be infected with AAV.
- Stable and long-lasting expression. The episome can be formed in the host cell and exist in the cell nucleus, and it can be continuously expressed for more than 5 months in the tissues where cell division is not vigorous.
- Strong diffusivity. rAAV has much higher diffusivity than AdVs and LVs and can penetrate the blood-brain barrier, which makes it the most ideal tool for neuronal and glial cell infections.
What We Offer to Develop Adeno-Associated Viral Vectors?
Selection of vector backbone
Our commonly used vector backbones include pAAV, pHelper pRC, etc. Among them, pAAV is responsible for carrying the target gene, pHelper contains the auxiliary genes required for AAV, and pRC is used for the replication and packaging of AAV.
Cloning of target gene
We insert the target gene into the pAAV vector by usually choosing a suitable restriction endonuclease to cleave the vector and the target gene, and then ligase is used to connect the target gene with the vector, and finally, the recombinant vector containing the target gene is obtained after transformation and screening.
Adding auxiliary genes
This step involves the co-transfection of auxiliary genes in pHelper with the recombinant vector into host cells to provide the auxiliary functions required for AAV replication and packaging.
Production and purification of AAV
Finally, we will culture the transfected host cells, and after a large amount of AAV is expressed, the AAV virus particles will be extracted from the cells by ultracentrifugation and other methods, and purified by filtration, centrifugation, concentration and other steps.
Our AAV vectors can be used for gene therapy studies (both in vivo and in vitro) for a wide range of diseases, and they are also widely used as characterization vectors for gene function studies, disease modeling, and the preparation of knockout mice. If you need technical support for AAV vector development in gene therapy, please feel free to contact us, we can provide one-stop AAV vector development service for you.
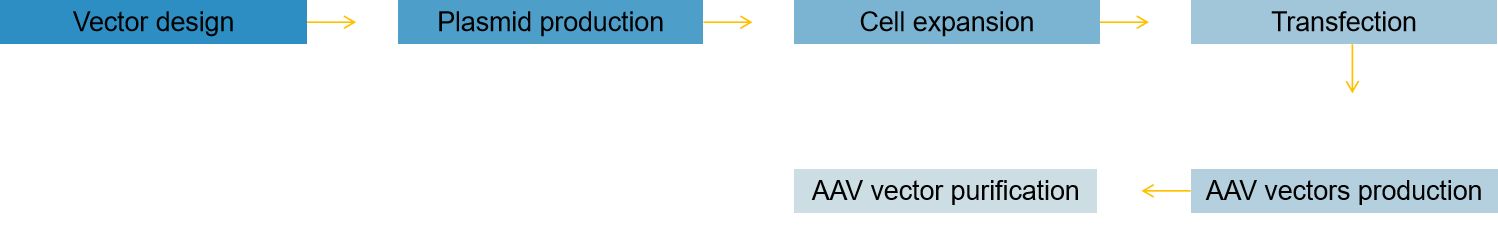 Fig.1 Our development process of AAV vectors. (CD Formulation)
Fig.1 Our development process of AAV vectors. (CD Formulation)
Applications of Our Adeno-Associated Viral Vector Development Service
- Adenoviral vectors as gene vaccines. Recombinant adenoviral vectors can be used to create various types of cancer vaccines by expressing tumor-associated antigens or by facilitating the process of tumor lysis. Antigens currently used to create cancer vaccines include, for example, the prostate cancer antigen PSA, the pancreatic cancer antigen CEA, and others.
- Adenoviral vectors in antitumor therapy. Since the p53 oncogenic pathway is dysfunctional in many tumor cells, adenoviral vectors can be used to re-express p53 to cause growth arrest or apoptosis in these tumor cells. Selectively replicating adenoviral vectors can specifically kill tumor cells through tumor lysis.
- Adenoviral vectors deliver a cellular "Suicide Gene". In addition to expressing the oncogene p53, adenoviral vectors can also kill tumor cells by expressing the transforming enzymes of certain precursor drugs.
- Adenoviral vectors deliver immunomodulatory genes. Adenoviral vectors can also be used to stimulate anti-tumor immune responses in patients by delivering and expressing immunomodulatory genes. For example, adenoviral vectors carrying interferon IFN-β or IFN-α-2b genes can be used to treat malignant pleural mesothelioma with a strong safety profile.
Our Platforms for Adeno-Associated Viral Vector Development
| Technologies & Platforms |
Content Description |
| Transient transfection technology platform |
We apply the transient transfection technology platform in AAV vector development, which involves the transient introduction of plasmid DNA into host cells for the production of AAV vectors. The technology platform is flexible and scalable and plays an important role in AAV vector development. By utilizing this technology platform, we can achieve more efficient and robust AAV production through continuous optimization of process parameters and adoption of advanced technologies. |
| Gene delivery technology platform |
In our AAV vector design, we focus on building a stable and efficient gene delivery platform. By selecting appropriate promoters, terminators and regulatory elements, etc., it ensures that exogenous genes can be precisely expressed in host cells. |
| Gene editing technology platform |
We provide gene editing technology that improves the precision of AAV vectors. We combine AAV with CRISPR-Cas technology to enable precise editing of target sites. Among other things, AAV is a major candidate vector for CRISPR-Cas, which can be used for therapeutic genome editing in vivo. |
| AI-AAV technology technology platform |
Compared with traditional directed evolution, AI-assisted screening of AAV has accomplished a multi-dimensional breakthrough. We utilize the AI-AAV platform for multiple research fields such as neurology and ophthalmology, aiming to develop gene therapy vectors with over 100 AAV neo-coat-shell sequences. |
Highlights of Our Adeno-Associated Viral Vector Development Service
- Custom development services. We offer customized development approaches to best meet the needs of investigators in gene therapy viral vector development.
- Quality assurance. Our professional research team has many years of development experience in gene therapy development, and our company performs strict quality control at every step from vector design to viral vector purification.
- One-stop development services. We offer a full range of AAV vector development services that can maximize the efficiency of our clients' research.
Published Data
Technology: Gene editing technology
Journal: Prog Retin Eye Res
IF: 17.8
Published: 2014
Results: Inherited retinopathies (IR) are common untreatable blinding conditions. Most of them are inherited as monogenic disorders, due to mutations in genes expressed in retinal photoreceptors (PR) and in retinal pigment epithelium (RPE). To date, recombinant vectors based on the adeno-associated virus (AAV) represent the most promising tool for retinal gene therapy, given their ability to efficiently deliver therapeutic genes to both PR and RPE and their excellent safety and efficacy profiles in humans.
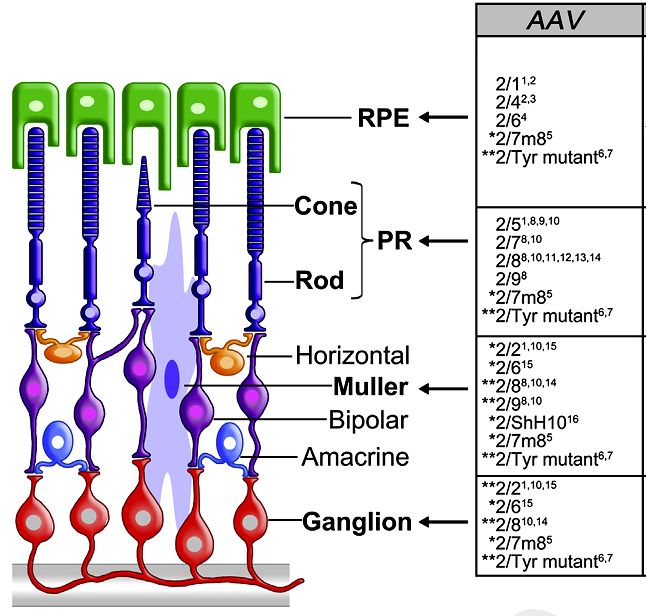 Fig.2 Retinal tropism of AAV. (Trapani I, et al., 2014)
Fig.2 Retinal tropism of AAV. (Trapani I, et al., 2014)
CD Formulation is an industry leader in the development of gene therapy formulations, and we have an experienced team of specialists and years of experience in gene therapy formulation development services. We have proven ourselves to be trustworthy with our services and products. If you are interested in us, please feel free to contact us.
Reference
- Trapani I, Puppo A, et al. Vector platforms for gene therapy of inherited retinopathies. Prog Retin Eye Res. 2014, 43:108-28.
Related Services

 Fig.1 Our development process of AAV vectors. (CD Formulation)
Fig.1 Our development process of AAV vectors. (CD Formulation) Fig.2 Retinal tropism of AAV. (Trapani I, et al., 2014)
Fig.2 Retinal tropism of AAV. (Trapani I, et al., 2014)