Gene Therapy Formulation Testing
Inquiry
Gene therapy formulation testing plays an integral role in ensuring the safety, identity, efficacy, purity, and quality of gene therapy products. CD Formulation is an industry leader in gene therapy formulation development testing and supports our clients with a combination of specialized knowledge, extensive experience, and exceptional service. We look forward to providing reliable and comprehensive testing for your gene therapy formulation development.
Importance of Gene Therapy Formulation Testing
- Safety assurance. The complexity of gene therapy agents far exceeds that of conventional drugs. Vector viral residual proteins may trigger an immune storm, and random insertion of exogenous genes may lead to cancer risk. Through various programs such as host cell DNA residue testing and replicative virus testing (RCL/RCA), we help our clients rule out potential toxic side effects and guard the safety red line.
- Validation of effectiveness. Indicators such as vector titer, transduction efficiency, and gene expression persistence directly affect the therapeutic effect. We use ddPCR, digital cell imaging, and other cutting-edge technologies to establish a full-process testing program from vector production to functional validation to ensure accurate delivery and stable expression of therapeutic genes.
- Compliance support. In response to the special regulatory requirements of the relevant regulatory agencies for the development of gene therapy formulations, we strictly follow the relevant guidelines and provide compliant testing reports, covering modules such as vector characterization, process residue testing, and stability studies.
Explore Our Gene Therapy Formulation Testing
Our comprehensive assay support for gene therapy formulation development includes but is not limited to, a range of assays such as viral titers, residues, sequencing, and biosafety.
For example, we offer viral titer testing to accurately assess the concentration and activity of viral vectors to ensure accurate dosing of subsequent gene therapy agents. Our viral capsid analysis helps to identify the proportion of empty capsids in viral particles, which is critical for optimizing production processes and increasing the amount of active viral particles. Residue detection in troubleshooting harmful substances left in the production process of gene therapy formulations, including but not limited to exogenous viruses, chemical residues, etc., to avoid these impurities from triggering immune responses or other adverse effects. In addition, using sequencing technology, precise analysis of the genome of viral vectors can be used to ensure its integrity and correctness, avoiding subsequent problems caused by mutations or deletions. Tests such as biosafety testing are used to ensure the safety of gene therapy products by conducting comprehensive safety assessments of gene therapy formulations, including testing for the presence of exogenous pathogens, viral replication capacity, and potential tumorigenicity.
It is important to mention that our services also cover other aspects of gene therapy formulation development analysis, which can be found in the entries listed below.
Our Process of Gene Therapy Formulation Testing
Testing of gene therapy formulations is a complex and systematic process that covers multiple stages from raw material to final product.
- At the raw material stage, cell banks used in the production process need to be thoroughly tested to confirm their type and exclude exogenous pathogens.
- In the unpurified stage, the titer of the viral vectors, gene insertion, detection of microbial contamination, and the presence of replicating viruses need to be verified.
- In the purified stage, we will analyze the genomic titer, infection titer, and null rate of the viral vectors in detail. For example, the genomic titer is detected by PCR, and the empty capsid rate is analyzed using analytical ultracentrifugation (AUC). At the same time, residues, such as host cell DNA, plasmid DNA, and instrumental enzymes, need to be detected to ensure that their levels are within safe limits.
- At the product manufacturing stage, we also need to conduct biosafety tests, including the replication ability of viral vectors, potential tumorigenicity, and so on.
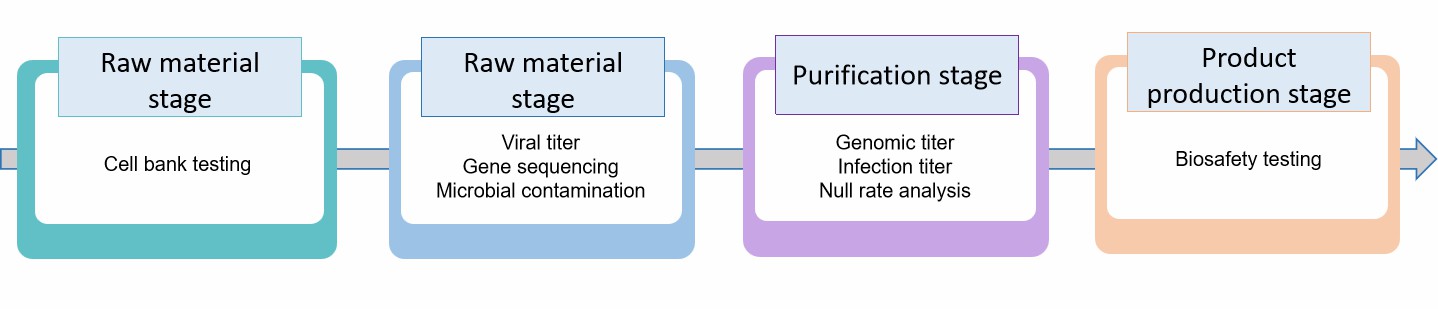 Fig.2 Our process of gene therapy formulation testing. (CD Formulation)
Fig.2 Our process of gene therapy formulation testing. (CD Formulation)
Our Platforms for Gene Therapy Formulation Testing
| Technologies & Platforms |
Content Description |
| Mass spectrometry technology platform |
We have established a comprehensive mass spectrometry detection technology platform for mass and sequence analysis of proteins. For example, for AAV coat protein, mass spectrometry analysis can help identify post-translational modifications, variant forms, and unknown structural features of the protein. |
| Viral vector quantitative detection technology platform |
We can rapidly and sensitively detect genomic RNA copy numbers in lentiviral vectors by quantitative reverse transcription polymerase chain reaction, which provides an effective means for quantification of viral vectors. |
| Sequencing technology platform |
We have established advanced sequencing technology platforms, such as HiFi sequencing, and nanopore sequencing technology. These technologies can be used to generate long-read lengths and play a critical role in viral vector discovery and design, host integration studies, and production optimization. |
Highlights of Our Gene Therapy Formulation Testing
- We have the scientific expertise to meet complex gene therapy formulation testing requirements.
- We have the regulatory knowledge and quality systems to support all testing needs.
- We provide continuous and transparent communication to ensure our clients are kept informed of project progress in a timely and accurate manner.
- We can provide customized testing services based on client requirements and characteristics.
As a leader in gene therapy formulation development, CD Formulation is committed to providing professional gene therapy formulation testing services. If you are interested in us, please feel free to contact us.
Related Services


 Fig.2 Our process of gene therapy formulation testing. (CD Formulation)
Fig.2 Our process of gene therapy formulation testing. (CD Formulation) 