Adeno-Associated Virus Empty Capsid Analysis
Inquiry
In the field of gene therapy, Adeno-associated virus (AAV) has attracted much attention as an effective gene vector. However, to ensure the safety and efficacy of gene therapy, accurate determination of the empty capsid rate in AAV preparation is required. CD Formulation has many years of experience in gene therapy formulation development, and we provide comprehensive technical support to help investigators advance the development of gene therapy formulations, including AAV capsid analysis. We have the advanced technology and expertise to accurately determine AAV empty capsid rates, which may include the use of advanced assays such as electron microscopy, flow cytometry, or PCR technology.
Why Analyze AAV Empty Capsid?
Gene therapy products, such as AAV products, generate a range of product-related impurities during the manufacturing process. These product-related impurities not only reduce the effectiveness of the product but also pose safety concerns such as immunogenicity. Therefore, it is necessary to utilize reliable analytical techniques to accurately control the capsid rate of AAV products to ensure the safety and efficacy of the products.
Our Services for AAV Empty Capsid Analysis
Protein analysis
Proteins in AAV preparations are analyzed using gel electrophoresis or mass spectrometry. By comparing the ratio of capsid proteins to vector proteins, the rate of empty capsids can be estimated.
Nucleic acid content analysis
The DNA content of the AAV preparation is determined using methods such as nucleic acid stains or PCR. Only a fraction of the DNA content corresponds to a truly effective vector compared to the total virus particles.
Electron microscopy observation
The morphology of AAV particles was observed using an electron microscope. The proportion of empty capsids can be roughly estimated by directly observing the morphological characteristics of the virus particles.
Functional assay
The proportion of effective vectors in AAV preparations is evaluated by functional assays such as cell infection assays. Since empty capsids lack gene loading, they do not exhibit specific functions when infecting cells.
Data analysis
Regardless of which method is used, accurate data analysis is required to determine the AAV empty capsid rate. When comparing data from experimental and control groups, possible confounding factors should be taken into account and analyzed using appropriate statistical methods.
Our Technologies for AAV Empty Capsid Analysis
| Platforms & Technologies |
Content Description |
| Enzyme-linked immunosorbent assay (ELISA) |
This method determines the titer of the capsid protein by coloring the substrate in an enzymatic reaction and quantifying it with a specific monoclonal antibody. |
| Transmission electron microscopy (TEM) |
We use an accelerated electron beam to pass through the sample to form a high-resolution image and distinguish between empty virus particles and solid virus particles containing the genome by negative staining. |
| Charge detection mass spectrometry (CDMS) |
This method is an emerging technique for determining capsid mass by measuring the mass-to-charge ratio and charge of intact capsids. |
Highlights of Our AAV Empty Capsid Analysis
- Quality control. We have strict quality control processes to ensure the accuracy and reproducibility of assay results.
- Customer service. We provide professional customer service to help our clients understand the importance of empty capsid rate determination and how to interpret the results.
- Customized service. We can customize our services to meet the specific needs of our clients.
- Advanced technology. We have established an advanced technology platform for the accurate determination of AAV capsid rate.
Published Data
Technology: Mass photometry (MP) analysis technology
Journal: Int J Mol Sci
IF: 5.6
Published: 2023
Adeno-associated virus (AAV) is one of the most commonly used vectors in gene therapy. During the AAV manufacturing process, in addition to the AAV capsid containing the target transgene, particles with little or no genetic material are produced. These by-products may have adverse health effects and are therefore considered as impurities. So far, analytical ultracentrifugation (AUC), transmission electron microscopy (TEM) and charge detection mass spectrometry (CDMS) have been used to detect these impurities. This researcher proposed a new detection technique, Mass photometry (MP), based on the problems of these commonly used detection methods, which serves as a rapid, label-free orthogonal technique for a wide range of serotypes without the need to adjust method parameters.
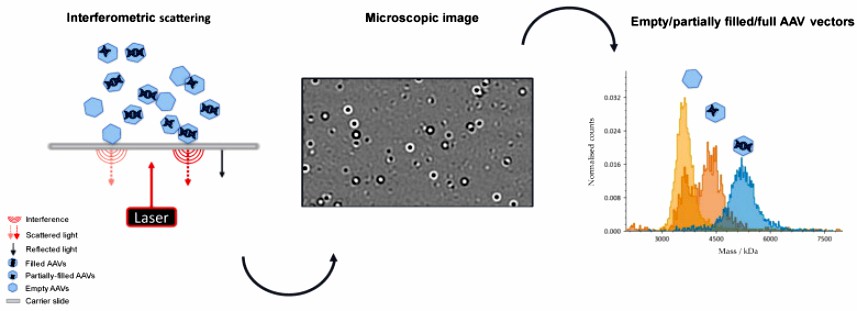 Fig.1 Schematic illustration of adeno-associated virus empty capsid mass photometry measurement. (Wagner C, et al., 2023)
Fig.1 Schematic illustration of adeno-associated virus empty capsid mass photometry measurement. (Wagner C, et al., 2023)
Accurate determination of AAV capsid rates is critical for gene therapy research and applications. CD Formulation is dedicated to helping researchers better understand the components of AAV formulations, thereby improving the safety and efficacy of gene therapy formulations. If you are interested in us, please feel free to contact us.
References
- Wagner C, et al. Quantification of Empty, Partially Filled and Full Adeno-Associated Virus Vectors Using Mass Photometry. Int J Mol Sci. 2023 Jul 3;24(13):11033.
Related Services

 Fig.1 Schematic illustration of adeno-associated virus empty capsid mass photometry measurement. (Wagner C, et al., 2023)
Fig.1 Schematic illustration of adeno-associated virus empty capsid mass photometry measurement. (Wagner C, et al., 2023)