Plasmid GMP Testing for Gene Therapy
Inquiry
Plasmids are small, circular DNA molecules that are commonly used to carry exogenous genes into host cells. Plasmids have an important role in gene therapy research as non-viral vectors, including injecting plasmid DNA into patients or using plasmid DNA to produce other therapeutic products such as viral vectors or mRNA. Plasmid GMP testing refers to a series of good manufacturing practice tests performed on plasmid DNA during the manufacturing process to ensure the safety and efficacy of gene therapy products.
CD Formulation aims to provide full technical support for gene therapy research to help researchers advance gene therapy research. We can provide a full range of plasmid GMP testing services to ensure gene purity and sequence accuracy.
Importance of Plasmid GMP Testing for Gene Therapy
- Quality assurance. GMP testing ensures that the plasmid DNA production process adheres to strict quality standards, including key quality attributes such as purity, concentration, stability and sterility.
- Risk reduction. GMP testing minimizes the risks associated with substandard products in clinical trials, such as contamination, cross-contamination, mix-ups and errors.
- Safety assurance. GMP testing includes microbiological safety testing of plasmid DNA, such as sterility testing, endotoxin testing, and mycoplasma testing, to ensure that the final product is safe and harmless to patients.
- Effectiveness validation. GMP testing also includes functional validation of plasmid DNA, such as gene expression efficiency and biological activity testing, to ensure that the product can perform as expected in clinical applications.
Our Services for Plasmid GMP Testing for Gene Therapy
Fermentation
Plasmid GMP testing covers a wide range of steps from strain library establishment, cell culture, lysis, and purification to formulation and filtration of the final product. The key tests we offer are shown below.
Plasmid screening
When used in the downstream production of biologics or viral vectors, plasmids are considered raw or starting materials. Because plasmids are produced by microbial production systems, there is a risk that plasmids may introduce contamination into the manufacture of a biopharmaceutical product. Before use in the manufacture of GMP biopharmaceutical products, we typically screen plasmids using microbiological methods, including, for example, sterility and mycoplasma testing.
Residue testing
Plasmid preparations should be free of residual host cell proteins and DNA, and we utilize ELISA and PCR methods that can be used for residue testing.
Sequence analysis
We utilize advanced sequencing technology platforms to sequence plasmids, which often includes sequence analysis to confirm the identity of the plasmid and to ensure the absence of sequence variation and potential cross-contamination.
Additional notes
As regulatory guidelines for plasmid quality in gene therapy continue to evolve. We may also utilize other methods such as concentration or copy number, homogeneity or restriction digestion.
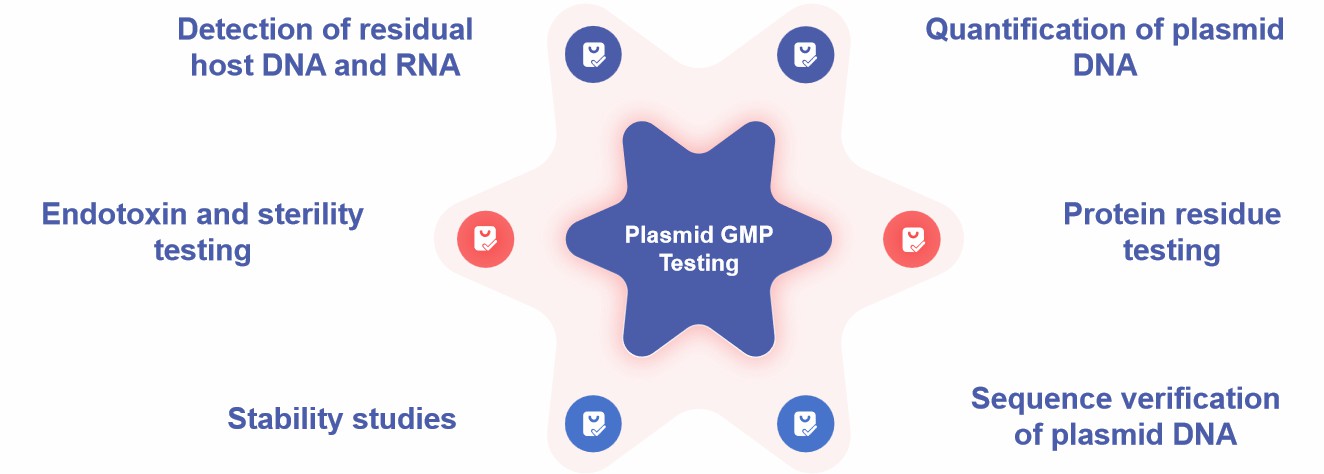 Fig.2 Plasmid GMP testing services. (CD Formulation)
Fig.2 Plasmid GMP testing services. (CD Formulation)
Our Platforms for Plasmid GMP Testing for Gene Therapy
| Platforms & Technologies |
Content Description |
| High-density fermentation technology |
By optimizing the fermentation conditions, high-density growth of engineering bacteria is achieved, thus increasing the yield of plasmid DNA. |
| Chromatography purification technology |
Purification of plasmid DNA using different chromatographic media to ensure high purity and low impurity level of the product. |
| Whole genome sequencing |
Whole-genome sequencing of plasmid DNA to ensure the integrity and accuracy of the genome, especially for genetically modified plasmids. |
| Quality control technology |
Includes testing of plasmid DNA for identification, content, purity, host cell DNA residue, transfection efficiency, endotoxin levels, and sterility to ensure product quality. |
Highlights of Our Plasmid GMP Testing for Gene Therapy
- We have well-established and advanced plasmid testing instrumentation to ensure that we provide accurate assay results.
- We specialize in biosafety and are able to quickly test and communicate with clients to ensure on-time delivery of test results.
- We have extensive testing experience and can provide our clients with specialized knowledge as well as sound scientific methodology.
- We deliver raw data results in addition to final analytical results.
Published Data
Technology: Plasmid DNA quality control technology
Journal: Methods Mol Biol
IF: 4.2
Published: 2022
The article discusses the growing interest in using circular DNA forms, such as plasmid DNA, for gene therapy and DNA vaccination. It highlights the importance of stability and quality control for clinical applications. The study focused on the long-term stability of GMP-grade pCMVβ plasmid DNA, which was stored at -20°C for 20 years and monitored continuously. Another plasmid, pCMV-Luc, stored for 15 years, was used as a control. The DNA's stability was evaluated using capillary gel electrophoresis (CGE) and its functionality was tested in vitro with a LacZ assay. The analysis revealed changes in the DNA's structure but maintained its ability to express genes, indicating that proper storage conditions can preserve the integrity of plasmid DNA for extended periods, which is crucial for long-term storage of reference samples.
CD Formulation provides professional and reliable plasmid GMP testing technical services to help researchers advance the development and application of gene therapy formulations. If you are interested in us, please feel free to contact us.
References
- Schmeer M, et al. Capillary Gel Electrophoresis (CGE) for Quality Control of Plasmid DNA in Gene Therapy: Quality Control of 20 Years Stored GMP-Grade Plasmid DNA. Methods Mol Biol. 2022, 2521:317-328.
Related Services


 Fig.2 Plasmid GMP testing services. (CD Formulation)
Fig.2 Plasmid GMP testing services. (CD Formulation)