Adeno-Associated Virus Tissue Distribution Identification
Inquiry
Adeno-Associated Virus(AAV) has become a popular gene delivery vector for gene therapy development. To test the reliability and safety of AAV vectors in vivo in early clinical studies of gene therapy formulations, one of the key issues is to characterize their distribution and retention time in multiple organs and tissues.
CD Formulation provides AAV tissue distribution characterization service to overcome the problem of AAV in gene therapy applications. With our advanced technology platform and experienced research team, we can help you obtain species-specific high-resolution AAV tissue distribution images.
Why Analyze AAV Tissue Distribution?
Presently available sub-organ AAV tissue dispersion identification methods often only provide an organ-level resolution, they do not address the various cell types at the sub-organ level. However, there isn't much research done on alternative administration methods, instead, these studies primarily concentrate on the intravenous injection of AAV. A bigger issue is that the majority of the studies carried out to yet have been on rodents, and the information gathered from these animals is not universally applicable to humans. There are now a lot of newly produced serotypes (both synthetic and natural) that lack comprehensive tissue distribution data. Therefore, our characterization and analysis of the tissue distribution of AAV is essential for selecting the most appropriate serotypes for subsequent studies, especially when the tissue distribution of multiple vectors needs to be compared. By using advanced analytical techniques, we can characterize the tissue distribution of different AAV gene vectors in the same species, significantly reducing the cost and duration of characterization.
Explore Our AAV Tissue Distribution Identification
The biodistribution of the AAV vectors we offer is critical for safety assessment in early clinical trials, especially in the case of direct in situ injection. By characterizing the distribution and retention time of AAV in vivo, the reliability and safety of clinical applications can be ensured.
We can provide our clients with high-resolution images of AAV tissue distribution using a variety of model organisms, including non-human primates, as well as various analytical methods, such as reporter gene fluorescence imaging, flow cytometry to isolate fluorescence-positive cells, etc. These services help to overcome some of the limitations of current research, such as having only organ-level resolution, lack of attempts at alternative routes of administration, and the problem that most experiments are performed on rodents.
The viral vector generation and purification
Packaging of AAV virus was performed using the appropriate cell culture system, followed by collection and purification of viral particles.
Preparation of animal models
Select the appropriate animal model and choose the appropriate injection route according to the research purpose, such as intravenous, intramuscular or intracerebral injection.
AAV injection and sample collection
Specific AAV vectors are injected into the animal model via the selected route. Tissue and organ samples are subsequently collected from the animals at specific time points.
Nucleic acid extraction and sequencing data analysis
DNA and RNA were extracted from the collected tissue samples and data analysis of sequencing data for multiple AAV tissue distributions.
Data analysis
Sequencing results were analyzed using statistical software and bioinformatics tools to determine the distribution pattern of AAV in different tissues.
Validation of results
The accuracy of the sequencing results was verified using other methods, such as qPCR, RT-PCR, and fluorescence imaging.
Our Technologies for AAV Tissue Distribution Identification
| Platforms & Technologies |
Content Description |
| qPCR assay technology |
This is an optimized qPCR method for detecting the distribution of AAV in preclinical biodistribution studies of gene therapy products with high sensitivity, specificity and accuracy. |
| Flow cytometry technology |
AAV-infected cells can be analyzed by flow cytometry to quantify the efficiency of AAV gene transduction. We assessed the tissue targeting of AAV by precisely measuring the transduction efficiency of AAV in different cell types. This method is characterized by high sensitivity in identifying AAV tissue distribution. |
| Luciferase activity assay technology |
We integrated a reporter gene encoding luciferase into an AAV vector and measured luciferase activity by infecting target cells. Using this technique, we can simply, rapidly, and quantitatively analyze the transduction efficiency of AAV in different tissues in a high-throughput manner, and thus assess its tissue distribution characteristics. |
Highlights of Our AAV Tissue Distribution Identification
- We can provide standardized testing services for gene therapy research clients and now have many years of experience supporting this type of service.
- We have extensive experience in supporting the development of gene therapy formulations from vector construction through to production.
- We have excellent service capabilities, such as laboratory facilities and state-of-the-art instrumentation. We can provide the fastest service in meeting the regulatory requirements for gene therapy products.
- Our professional R&D team and advanced technology platform ensure that we provide high-quality and reliable AAV tissue distribution identification services to our customers.
Published Data
Technology: Plasmid DNA-loaded nanoliposomes development
Journal: Hum Gene Ther
IF: 3.9
Published: 2022
Dravet syndrome is a severe neurological disorder caused by a defect in the SCN1A gene, which is crucial for the function of a specific type of sodium channel important in the brain's inhibitory neurons. Researchers have developed a gene therapy using an adeno-associated virus (AAV9) to target and increase the activity of this gene specifically in these neurons. In a mouse model of the disease, this therapy reduced seizures and improved survival. Testing in nonhuman primates showed the therapy was well distributed throughout the brain and safe, with no adverse effects. These findings suggest the potential of this targeted gene therapy as a treatment for Dravet syndrome.
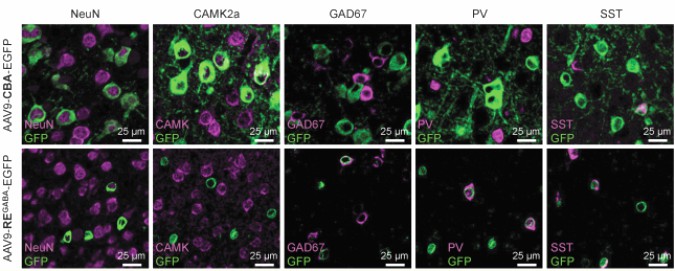 Fig.1 Representative images of EGFP colocalization with neuronal markers. (Tanenhaus A, et al., 2022)
Fig.1 Representative images of EGFP colocalization with neuronal markers. (Tanenhaus A, et al., 2022)
CD Formulation provides superior technical support and comprehensive solutions for gene therapy formulation development. We aim to advance research progress in gene therapy formulation development by collaborating with researchers together. If you are interested in us, please feel free to contact us.
References
- Tanenhaus A, et al. Cell-Selective Adeno-Associated Virus-Mediated SCN1A Gene Regulation Therapy Rescues Mortality and Seizure Phenotypes in a Dravet Syndrome Mouse Model and Is Well Tolerated in Nonhuman Primates. Hum Gene Ther. 2022, 33(11-12):579-597.
Related Services

 Fig.1 Representative images of EGFP colocalization with neuronal markers. (Tanenhaus A, et al., 2022)
Fig.1 Representative images of EGFP colocalization with neuronal markers. (Tanenhaus A, et al., 2022)