In gene therapy products using viral vectors, the titer indicates the product content and the amount of virus. Viral titers are categorized into different types such as total particle number, genomic titer, infectious titer, and transduction titer. CD Formulation is dedicated to the development of gene therapy formulations. We specialize in providing comprehensive and professional technical support for the development of gene therapy formulations, including virus titer testing, related testing and quality control service programs.
Types of Viral Titer Testing
Total particle number and genomic titer indicate the physical number of viruses and are physical titers. Infectious titer and transduction titer indicate the number of biologically active viruses and are biological titers. We can provide testing services for total particle number and transduction titer.
- Physical titer (total particle count). Refers to the total number of physical virus particles.
- Biological titer (transduction titer). The transduction titer refers to the number of viruses that can effectively transduce the target cells and express the transgene product, which is more reflective of the functional content indicator of the active ingredient of the gene therapy product.
Why Test Viral Titer?
- Ensure the accuracy of the formulation dosage. Accurate titer tests can improve treatment outcomes by ensuring that patients receive the appropriate therapeutic dose and avoid under-under or over-dosing.
- Quality control. Titer tests are a critical step in assessing the quality of the virus preparation, helping to monitor the consistency of the virus batch to batch and ensuring product quality and safety.
- Avoiding immune reactions and toxic effects. Appropriate viral titers can reduce immune responses and potential toxic effects in patients and improve the safety of therapy.
- Research and development. During the research and development phase of gene therapy, titer tests help determine the potency of the viral vector and the optimal delivery strategy.
Our Services for Viral Titer Testing
Adeno-associated virus (AAV) is a common viral vector for carrying and delivering therapeutic genes in gene therapy. Its titer assay is important in assessing the number of viral particles in AAV vector preparation. We currently use qPCR, digital PCR, enzyme-linked immunosorbent assay (ELISA), and cellular infection assay for AAV titer measurement. The specific choice of method needs to be decided based on the customer's actual experimental needs, the characteristics of the viral samples, and other factors. Below is a brief process we use when determining AAV titers.
Sample preparation
We first need to prepare an AAV sample, which can be diluted appropriately for the AAV sample to be tested.
PCR reaction
Using an appropriate primer design, a PCR reaction is performed to amplify the AAV genome from the samples.
Standard curve construction
A series of PCR reactions with known concentrations of AAV standard samples can be performed simultaneously to generate a standard curve.
Measurement of samples
The PCR reaction product is compared with the standard curve to determine the copy number of the AAV genome in the sample.
Calculate titer
Based on the results of the PCR reaction, combined with the dilution of the sample, the titer of the AAV virus is calculated.
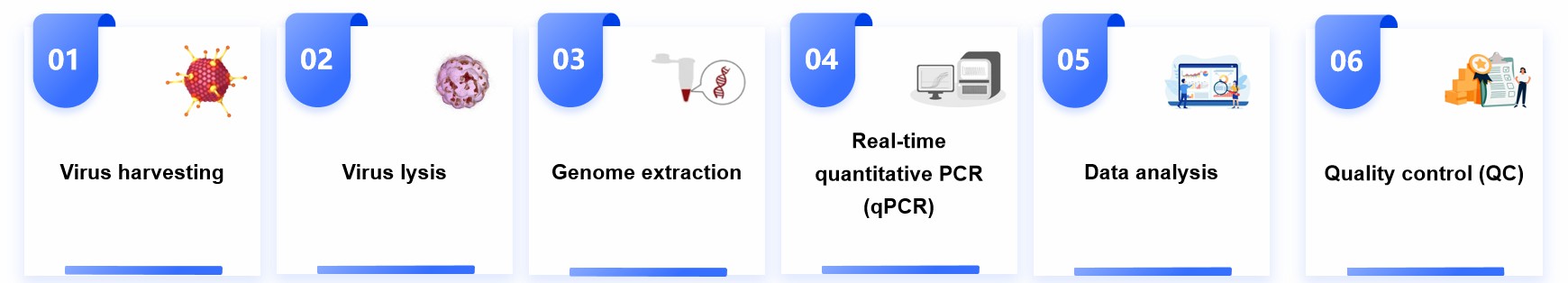 Fig.1 Adeno-associated virus titer testing. (CD Formulation)
Fig.1 Adeno-associated virus titer testing. (CD Formulation)
Our Technologies for Viral Titer Testing
| Platforms & Technologies |
Content Description |
| Droplet digital PCR (ddPCR) |
As a highly sensitive and accurate quantification technique, ddPCR disperses the sample into thousands of microdroplets by microfluidics, each of which contains a very small amount of DNA molecules, enabling absolute quantification at the single-molecule level. This method is not dependent on a standard curve, providing greater accuracy and reproducibility, and is particularly suitable for titer determination of viral vectors such as AAV. |
| Virus quantification by capillary electrophoresis |
This is a technique that utilizes capillary electrophoresis to separate virus particles and detect them optically or chemically. It provides information on the size distribution and concentration of viral particles and helps to assess the purity and homogeneity of viral preparations. |
| qRT-PCR |
By quantitative reverse transcription polymerase chain reaction, the qRT-PCR method can quickly and sensitively detect genomic RNA copy numbers in lentiviral vectors, providing an effective means of quantifying viral vectors. |
Highlights of Our Viral Titer Testing
- We have a professional team based on chief scientists, industry experts, and excellent master and doctoral talents.
- We have an advanced R&D platform for gene therapy formulations and have provided development services for many enterprises and high efficiency.
- We adhere to the attitude of continuous innovation to create value for customers and contribute to human health.
- We can provide customized viral titer assay solutions according to the customer's project characteristics and requirements.
Published Data
Technology: rAAV infectious titer testing
Journal: Hum Gene Ther
IF: 3.9
Published: 2023
The article discusses the use of recombinant adeno-associated virus (rAAV) for gene therapy in treating various diseases and the need for reliable analytical methods to support this therapy. The TCID50 assay, which measures AAV infectivity, is traditionally difficult due to its variability. The study presents an optimization of this assay by using droplet digital PCR (ddPCR) for endpoint detection instead of the standard quantitative PCR (qPCR). Over 18 independent runs, the optimized method with ddPCR showed better interassay precision. The addition of a secondary threshold for scoring infectivity further improved precision. The study concludes with an optimized TCID50 method that enhances precision in rAAV infectious titer testing, which is crucial for process development and manufacturing in gene therapy.
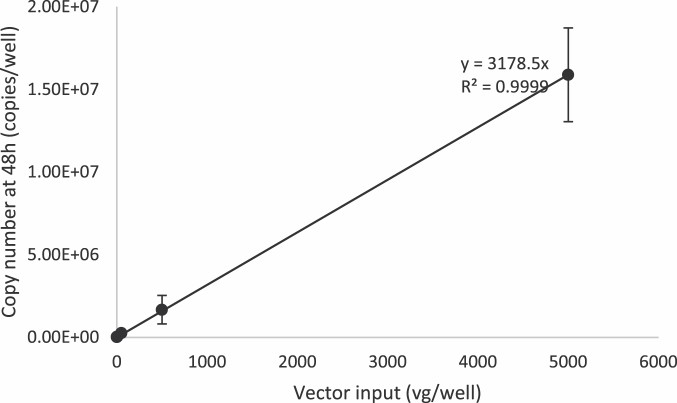 Fig.2 Identification of adeno-associated viruses-2 viral genome replication using the ddPCR method. (Duong T, et al., 2023)
Fig.2 Identification of adeno-associated viruses-2 viral genome replication using the ddPCR method. (Duong T, et al., 2023)
CD Formulation offers a viral titer testing service that plays an important role in gene therapy research for measuring viral load and virulence. If you are interested in us, please feel free to contact us.
References
- Duong T, et al. Improvement of Precision in Recombinant Adeno-Associated Virus Infectious Titer Assay with Droplet Digital PCR as an Endpoint Measurement. Hum Gene Ther. 2023, 34(15-16):742-757.
Related Services


 Fig.1 Adeno-associated virus titer testing. (CD Formulation)
Fig.1 Adeno-associated virus titer testing. (CD Formulation) Fig.2 Identification of adeno-associated viruses-2 viral genome replication using the ddPCR method. (Duong T, et al., 2023)
Fig.2 Identification of adeno-associated viruses-2 viral genome replication using the ddPCR method. (Duong T, et al., 2023)