Adeno-Associated Virus Capsid Protein Identification
Inquiry
Capsid protein plays a crucial role in gene therapy. They are major components of viral vectors such as adeno-associated virus (AAV) and determine the packaging efficiency, delivery properties, immunogenicity, and tissue specificity of viral particles. The analysis of AAV capsid protein is critical for optimizing viral vector design and improving the safety and efficacy of gene therapy drugs.
CD Formulation aims to provide comprehensive services for developing gene therapy agents, especially in identifying AAV capsid protein. Our reliance on a range of cutting-edge technology enables us to evaluate the caliber of AAV preparations, guaranteeing the security and effectiveness of gene therapy medications.
Why Identify AAV Capsid Protein?
- Ensure the quality of viral vectors. AAV capsid protein is a major component of viral particles, which determines the packaging efficiency and delivery properties of AAV. Rapid and efficient analysis of AAV capsid protein can optimize the design of viral vectors and improve the safety and efficacy of gene therapy drugs.
- Promoting gene therapy product development. The identification of AAV capsid protein is an important step in the development and commercialization of gene therapy products. We analyze AAV capsid proteins for complete protein mass counts to facilitate the development of gene therapy products.
- Improving gene therapy targeting and transduction efficiency. By modifying AAV capsid protein, the targeting and transduction efficiency of gene therapy can be improved.
- Reduced immunogenicity. Modification of AAV capsid protein can reduce its immunogenicity and reduce the effect of pre-existing immunity on gene therapy effect in vivo.
- Improvement of productivity. AAV capsid protein has a great impact on the yield of rAAV. By optimizing the structure of the coat protein, the yield of rAAV can be increased and the production cost can be reduced.
Our Services for AAV Capsid Protein Identification
Protein concentration and stoichiometric ratio analysis
The concentration of VP1, VP2 and VP3 proteins in the AAV coat and the molar ratios between them, which are critical for predicting the infectivity and stability of AAV vectors, were determined by techniques such as Simple Western, HPLC, SEC-MALS, etc.
Protein purity and integrity analysis
SDS-PAGE, western blot and other methods were used to detect the purity and integrity of AAV capsid protein and assess the quality of AAV preparation
Serotype identification
Identify serotypes of structural protein sequences by liquid mass spectrometry platform to ensure the correct serotype of AAV used.
Empty capsid detection
Techniques such as AUC (Analytical Ultracentrifugation) are utilized to quantify the amount of DNA in the AAV capsid and to assess the percentage of empty capsids, which is critical to the safety and efficacy of AAV products.
Post-translational modification analysis
Mass spectrometry (MS) is used to identify post-translational modifications, such as acetylation and phosphorylation, of AAV capsid proteins, which are important to help researchers understand the biological properties of virus particles.
Analysis of capsid protein variants
LC-MS allows the identification of variants of AAV capsid protein, including different forms of capsid protein due to post-translational modifications or proteolysis.
Biological activity analysis
We assessed the biological activity of AAV capsid protein by evaluating the titer of infection and the expression of target proteins, which are essential for demonstrating the effectiveness of AAV as a gene therapy vector.
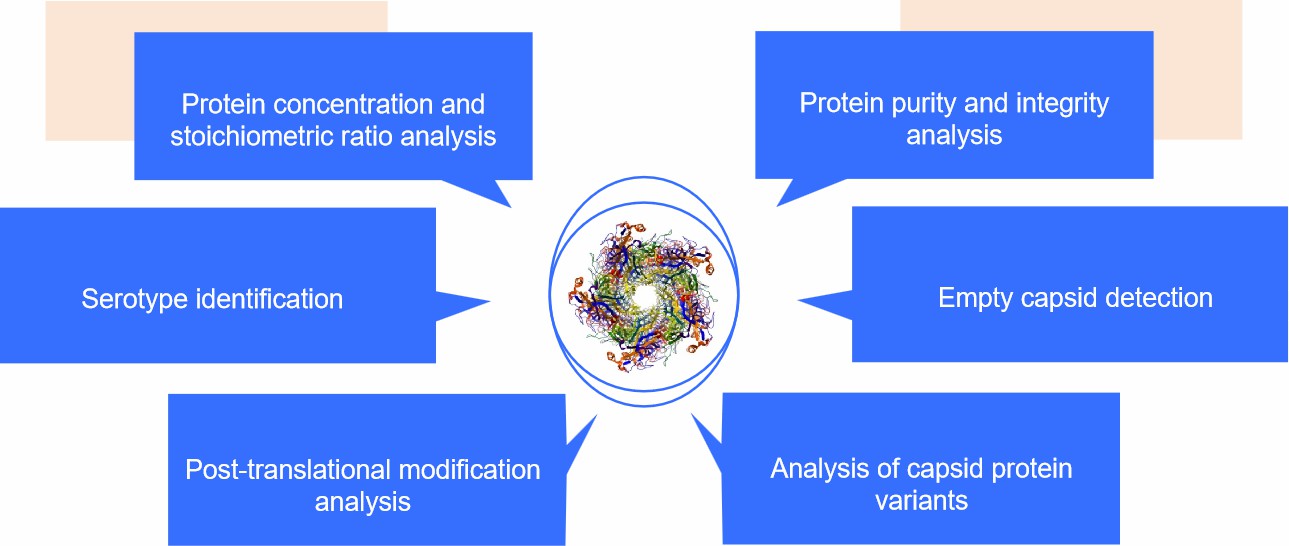 Fig.2 Adeno-associated virus capsid protein analysis. (CD Formulation)
Fig.2 Adeno-associated virus capsid protein analysis. (CD Formulation)
Our Technologies for AAV Capsid Protein Identification
| Platforms & Technologies |
Content Description |
| Protein gel electrophoresis (SDS-PAGE) |
This method can be used to test the purity and integrity of AAV capsid protein. Protein bands can be quickly identified and the quality of AAV preparations can be assessed by running samples through a PAGE gel and staining them. This method is simple and inexpensive and is often used for preliminary capsid protein analysis. |
| Western blotting (WB) |
This technique allows for the detection and semi-quantitative analysis of specific proteins. Using antibodies specific for AAV capsid proteins, the presence, molecular weight, and relative abundance of the protein can be accurately determined, making it invaluable for both qualitative and quantitative analysis of capsid proteins. |
| High-performance liquid chromatography (HPLC) |
HPLC is a highly efficient technique for analyzing protein purity, especially in the separation of different forms of AAV capsid protein using specific columns. HPLC is a highly efficient technique for analyzing the purity of AAV, especially in the separation of different forms of AAV capsid protein using specific columns. |
| Mass spectrometry (MS) |
Mass spectrometry method is a highly precise means of protein analysis for mass and sequence analysis of proteins. For AAV capsid proteins, mass spectrometry can help to identify post-translational modifications, variant forms and unknown structural features of the protein, which is very important for a deeper understanding of the biological properties of the virus particles. |
| Enzyme-linked immunosorbent assay (ELISA) |
ELISA is a rapid method for quantification of capsid proteins, utilizing antibodies that have a high degree of specificity and affinity for the proteins, allowing rapid detection of specific AAV capsid proteins. |
Highlights of Our AAV Capsid Protein Identification
- Standardized operation process. We have established standardized experimental procedures and operation guidelines to ensure consistency and reproducibility of each experimental operation.
- Use of advanced analytical techniques. We use high-sensitivity and high-resolution analytical techniques to improve the accuracy of detection.
- Professional staff training. We regularly provide technical and theoretical training to our laboratory personnel to ensure that they have a deep understanding of the identification techniques of AAV coat protein and can operate skillfully.
- Data management. We have established accurate, state-of-the-art data recording and analysis systems to ensure that we provide data integrity and traceability.
Published Data
Technology: Plasmid DNA-loaded nanoliposomes development
Journal: Hum Gene Ther
IF: 3.9
Published: 2022
The article discusses adeno-associated viruses (AAVs) as valuable vectors for gene therapy and gene expression research. It highlights how engineered AAV variants have expanded their applications by modifying their properties. The study compared 50 commonly used AAVs across various cell types, including those derived from adipose tissue, liver, lung, brain, and eyes. The comparison utilized high-content imaging to assess efficiency, with results accessible through a web app. One AAV variant demonstrated effectiveness in a liver fibrosis model. The analysis concluded that a select few AAV variants (AAV2.NN, AAV9-SLRSPPS, AAV6.2, AAV6TM, and AAV1P5) were particularly efficient across all tested cell types, suggesting they could be a universal tool for gene modulation in cellular systems.
CD Formulation is committed to the development of gene therapy formulations. We are customer-oriented and continue to provide our customers with professional and comprehensive technical support services. If you are interested in us, please feel free to contact us.
References
- Weinmann J, et al. Identification of Broadly Applicable Adeno-Associated Virus Vectors by Systematic Comparison of Commonly Used Capsid Variants In Vitro. Hum Gene Ther. 2022, 33(21-22):1197-1212.
Related Services


 Fig.2 Adeno-associated virus capsid protein analysis. (CD Formulation)
Fig.2 Adeno-associated virus capsid protein analysis. (CD Formulation)