Gene Therapy Formulation Sequencing
Inquiry
Sequencing technology is an important tool in genomics, and it plays a key role in gene therapy product development. Sequencing technology reveals the structure and function of genetic information by analyzing the base sequences of DNA or RNA. In the development of gene therapy agents, sequencing technology is used throughout all stages from basic research to subsequent application, providing key data support.
CD Formulation provides comprehensive technical support for the development of gene therapy agents. In particular, our long-read, long-sequencing, and next-generation sequencing technologies play an indispensable role in ensuring the safety and correctness of gene therapy vectors for the development of gene therapy formulations.
Technical Advantages of Our Gene Therapy Formulation Sequencing
- High-throughput sequencing technology. We have adopted high-throughput sequencing technology (NGS), which has demonstrated high efficiency and low cost in sequencing for gene therapy formulation development and can complete large-scale genome sequencing in a short time.
- Long read long sequencing. We offer long-read, long-sequencing, which is critical for the precise assembly of genomes and the detection of structural variants. This technology reduces gaps in genome splicing and provides more complete genomic information.
- Micro-sample sequencing. Our sequencing technology platform allows for the processing of micro samples, helping clients save more experimental samples, which is especially important for the utilization of precious samples.
- Full-length transcript sequencing. Your technology-specific full-length transcript library construction methods enable access to full-length transcript sequences, which are critical for studying variable splicing and functional analysis of genes.
- Complex genome sequencing solutions. We use a combination of small fragment library sequencing, as well as multiple library sequencing and a variety of advanced sequencing platforms to obtain long-read long sequence data, integrate second-generation sequencing data, and assemble a fine map of the genome to provide customers with more accurate and detailed sequencing results.

Explore Our Gene Therapy Sequencing
Our reliable, state-of-the-art, accurate sequencing can provide strong technical support for the development of gene therapy agents and advance research in the field of gene therapy. Our sequencing has the distinct advantage of being able to sequence the entire structure of complete large gene fragments with high precision. This ability to obtain full length is important for the discovery and identification of fragment products, and high precision is critical for identifying unwanted mutations. In addition, we can also provide customized sequencing solutions to maximize our clients' research projects.
We customize gene therapy formulation sequencing services
Long-read sequencing technology offers transformative insights across various stages of AAV vector research, development, and production. For vector discovery, it enables highly accurate, long-read DNA sequencing, capturing comprehensive genome structures of AAV capsids, including intricate gene modules and repeats, to facilitate the identification of novel capsids. In vector design, this technology uncovers crucial details such as packaging anomalies and genomic truncations, improving safety and therapeutic efficacy by optimizing gene chimerization and splicing. It also plays a pivotal role in host integration studies by providing detailed molecular data on integration sites, frequencies, and mechanisms, helping researchers assess risks like genomic instability and refine vector safety. Additionally, during AAV production, long-read sequencing ensures genome integrity, monitors for contaminants, and enhances production cell lines, enabling more efficient and consistent manufacturing of high-quality viral particles. This end-to-end application underscores its importance in advancing gene therapy innovation.
Next-generation sequencing (NGS) technology plays a pivotal role in the quality testing of adeno-associated virus (AAV) vectors. NGS enables comprehensive AAV sequence analysis by facilitating whole genome or targeted region sequencing, ensuring accurate determination of the viral genome while assessing product purity, stability, and consistency. Additionally, NGS supports integration site analysis, a critical step in evaluating the safety of AAV therapies by detecting and identifying sequences where the vector may integrate into the host genome. Impurity detection is another vital application, with NGS identifying contaminants such as exogenous DNA, bacteria, and fungi that could compromise therapeutic efficacy or safety. Beyond standard analyses, specialized NGS-based services address complex testing needs, including capsid protein identification and residual DNA analysis. By leveraging NGS, AAV quality testing not only ensures product safety and effectiveness but also advances vector design, contributing to more efficient and secure gene therapy solutions.
Our Phase of Gene Therapy Sequencing
- Discovery phase. Gene therapy sequencing technologies are important in the viral vector discovery phase and can help researchers discover novel adeno-associated virus (AAV) vectors, for example. Utilizing high-throughput sequencing technology services helps to assist clients with in-depth discovery of the genetic diversity of natural AAV serotypes and engineered variants in order to develop candidate vectors with high transduction efficiencies and low immunogenicity. This phase lays the groundwork for selecting the best vectors for specific therapeutic needs.
- Design phase. Gene therapy sequencing refines vector development by analyzing gene constructs and optimizing their structure. Utilizing our advanced sequencing technology assesses the fidelity of the vector genome and ensures the correct assembly of each regulatory element of the gene. Additionally, sequencing technology helps to identify vectors designed with greater stability, packaging efficiency, and transgene expression profiles, thereby increasing the potential of their gene therapy formulations.
- Validation phase. Validation by sequencing ensures the safety and efficacy of gene therapy products and detects truncations in the vector genome that may affect therapeutic efficacy. Sequencing technology can also identify impurities that may be present during the manufacturing process, such as plasmid DNA or host cell contaminants. In addition, sequencing can detect host genome integration events, a potential safety concern associated with insertion mutagenesis. Comprehensive sequencing-based validation ensures the integrity, purity, and safety of the final gene therapy product, meeting stringent regulatory standards.
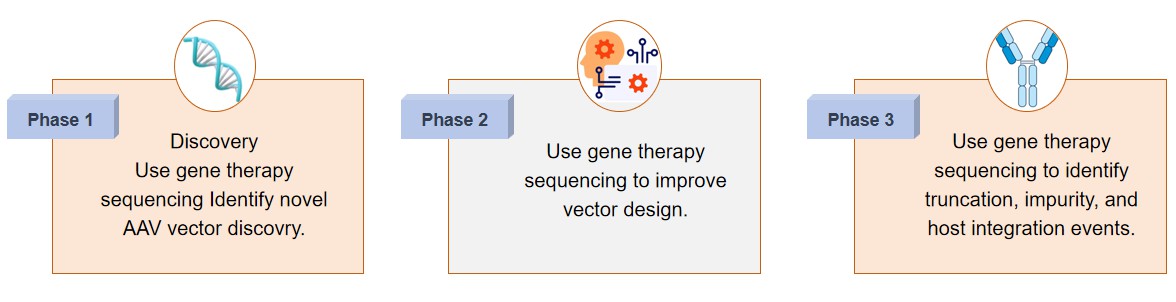 Fig.2 Phase of gene therapy sequencing. (CD Formulation)
Fig.2 Phase of gene therapy sequencing. (CD Formulation)
Our Platforms for Gene Therapy Sequencing
| Platforms & Technologies |
Content Description |
| HiFi sequencing technology |
HiFi sequencing, a cutting-edge single-molecule real-time (SMRT) technology, enables the generation of exceptionally long read lengths, reaching up to 20,000 base pairs. This capability is transformative for fully sequencing the AAV genome. It plays a crucial role in AAV vector discovery and design, host integration studies, and optimizing production processes. By uncovering challenges that traditional methods often miss, HiFi sequencing has facilitated breakthroughs such as identifying novel AAV capsids, enhancing vector designs, and gaining deeper insights into host integration events. Additionally, it supports robust comparative analyses across production platforms, driving advancements in AAV-based gene therapy development. |
| Nanopore sequencing technology |
Nanopore sequencing technology stands out for its exceptional ability to deliver ultra-long read lengths, enabling real-time analysis of individual DNA molecules. This capability makes it particularly well-suited for macro-genomics research and the sequencing of intricate genomic regions. In the context of AAV gene therapy, Nanopore technology has proven invaluable in uncovering high-resolution insights. |
Highlights Our Gene Therapy Sequencing
- Cost-effective gene sequencing. Our speed-of-sequencing features significantly reduce the cost of gene sequencing, making research and treatment more economical and lowering the barrier to entry.
- Rapid response to customer needs. Our rapid delivery capabilities enable us to respond quickly to customer needs and provide timely data support for the development of gene therapy agents.
- Advanced synthesis technology. We have mastered the technology of synthesizing difficult sequences, including repetitive sequences and high GC content sequences, to ensure the accuracy and reliability of synthesis.
- Dual testing to guarantee quality. We provide dual testing of enzymatic digestion and sequencing, and ensure the correct sequence by sequencing technology, which guarantees the high-quality standard of our products.
Published Data
Technology: Gene sequencing technology
Journal: Nucleic Acids Res
IF: 14.9
Published: 2011
Human genetic diseases have been successfully corrected by integrating functional copies of defective genes into human cells, but in some cases, the integration of therapeutic vectors activates proto-oncogenes. As a result, a large number of efforts have focused on analyzing integration site populations in patient samples, but the most commonly used methods for recovering newly integrated DNA suffer from severe recovery bias. In this article, the authors demonstrate a new phage-based method to easily and consistently recover integration site sequences in a form that can be analyzed directly using DNA barcoding and pyrophosphate sequencing, a technique that allows for simple estimation of the relative abundance of genetically modified cells in human gene therapy subjects and is critical for the detection of clonal expansions of cells that may be precursors to adverse events.
CD Formulation offers sequencing technology that provides reliable technical support for multiple stages of gene therapy formulation development. If you are interested in us, please feel free to contact us.
References
- Brady T, et al. A method to sequence and quantify DNA integration for monitoring outcome in gene therapy. Nucleic Acids Res. 2011, 39(11):e72.
Related Services


 Fig.2 Phase of gene therapy sequencing. (CD Formulation)
Fig.2 Phase of gene therapy sequencing. (CD Formulation)