Nanoformulation Biological Evaluation
Inquiry
It is essential to assess the biological impact of nanoformulations to create effective formulations and make informed clinical choices. CD Formulation stresses the significance of biologically evaluating nanoformulations, providing services such as hemolysis testing, cellular uptake assays, safety assessments, and research on pharmacokinetics and biodistribution. This assessment can help inform clinical practices, including formulation development, dosage decisions, and administration techniques.
Biological Characteristics of Nanoformulations
The biological characteristics of nanoformulations are as follows:
- Strong ability to penetrate biological membrane barriers and can pass through a variety of biological membrane barriers. For example, the gastrointestinal mucosal barrier greatly improves the bioavailability of poorly soluble drugs. The blood-brain barrier (BBB) increases brain drug levels and enhances efficacy. Blood-eye barrier, placental barrier, etc.
- Tissue and organ selectivity. By changing the particle size, surface structure or composition of nanoformulations, it promotes the enrichment of drugs to diseased tissues, improves drug efficacy, and reduces toxic side effects. Such as tumor targeting, lung targeting, etc.
- Obvious controlled release characteristics. Different nanoformulations have different structural compositions, but the properties of slow drug release as a drug reservoir are the same.
Our Nanoformulation Biological Evaluation Services
As a world-class nanoformulation development and manufacturing service company, we have rich experience in nanoformulation biological evaluation for years, focusing on nanoformulation hemolysis testing, nanoformulation cellular uptake assay, nanoformulation non-clinical safety assessment, nanoformulation pharmacokinetic studies and nanoformulation biodistribution studies.
Our Nanoformulation Biological Evaluation Methods
Due to the specific physicochemical properties, distribution and metabolic processes of nanoformulations, their physicochemical properties, distribution, and metabolic processes need to be considered during the in vivo and in vitro biological evaluation of nanoformulations. CD Formulation is committed to exploring and researching some appropriate nanoformulation biological evaluation methods, such as transmission electron microscopy, backscattered electron imaging, fluorescent labeling method, and isotope tracing methods.
Transmission Electron Microscopy
Transmission electron microscopy is the preferred method for studying nanoformulation cellular uptake. In addition to detecting the precise positioning of nanoparticles within cells, it can also provide details of the interaction between nanoparticles and cells. Due to its high resolution, it can also be clearly seen in organelles, cell invagination, and vesicle formation. This allows an in-depth study of the cellular uptake process, which is key to understanding the impact of nanoformulation surface characteristics on organisms.
Backscattered Electron Imaging
Backscattered electron imaging has been successfully applied to the nanoformulation biological evaluation. This technology is an electron imaging technique based on a scanning electron microscope. Backscattered electrons can focus on the surface morphology and atomic number distribution of the sample.
Fluorescence labeling
We determine the location and transport process of nanoformulations in biological systems by detecting fluorescence intensity.
Isotope Tracing
In order to obtain an intuitive and dynamic process of nanoformulations in the body, we used isotope tracing methods to detect the transfer and excretion of nanoformulations in the body.
Nanoformulation biological evaluation, whether in vivo or in vitro, has its own evaluation system and evaluation methods. Nanoformulations have special surface characteristics and in vivo uncertainties. Therefore, we need to fully evaluate the evaluation methods and means before conducting a biological evaluation of nanoformulations.
Why Choose Us for Nanoformulation Biological Evaluation?
- We have a lot of experience in nanoformulation biological evaluation, including nanoformulation hemolysis testing, cellular uptake assay, non-clinical safety assessment, pharmacokinetic and biodistribution studies.
- We have explored and developed nanoformulation biological evaluation methods, such as transmission electron microscopy, backscattered electron imaging, fluorescent labeling method and isotope tracing method.
- Our technical team has professional knowledge in nanoformulation, nanoformulation pharmacokinetics, nanoformulation toxicology, etc., and can respond to your nanoformulation biological evaluation needs in a timely manner and provide you with personalized professional technical services.
Published Data
Technology: Data modeling technology based on machine learning ensemble method
Journal: Journal of Polymers and the Environment
IF: 5.3
Published: 2024
Results:
The authors synthesized PEGylated konjac gum-loaded rosin pentaerythritol nanocomposites (KG/PEG/RE PNCs) using an environmentally friendly sonochemical approach to explore their potential antibacterial and antifungal properties against a range of pathogens including Candida albicans, Escherichia coli, Pseudomonas aeruginosa, Aspergillus brasiliensis, and Staphylococcus aureus. The authors modeled the complex dynamics affecting the viscosity of PNCs through an ensemble of machine learning (polynomial regression) to facilitate the understanding of the PNC behavior in relation to the synthesis parameters. The model facilitated the precise formulation of a formula to predict the viscosity of PNCs with high accuracy. The PNCs exhibited broad-spectrum antimicrobial activity, reaching an inhibitory plateau within the first week, confirming their efficacy as multifunctional antibacterial and antifungal agents. This integrated approach, combining advanced data modeling techniques with biological evaluations, will help optimize the further development of polymer nanostructures.
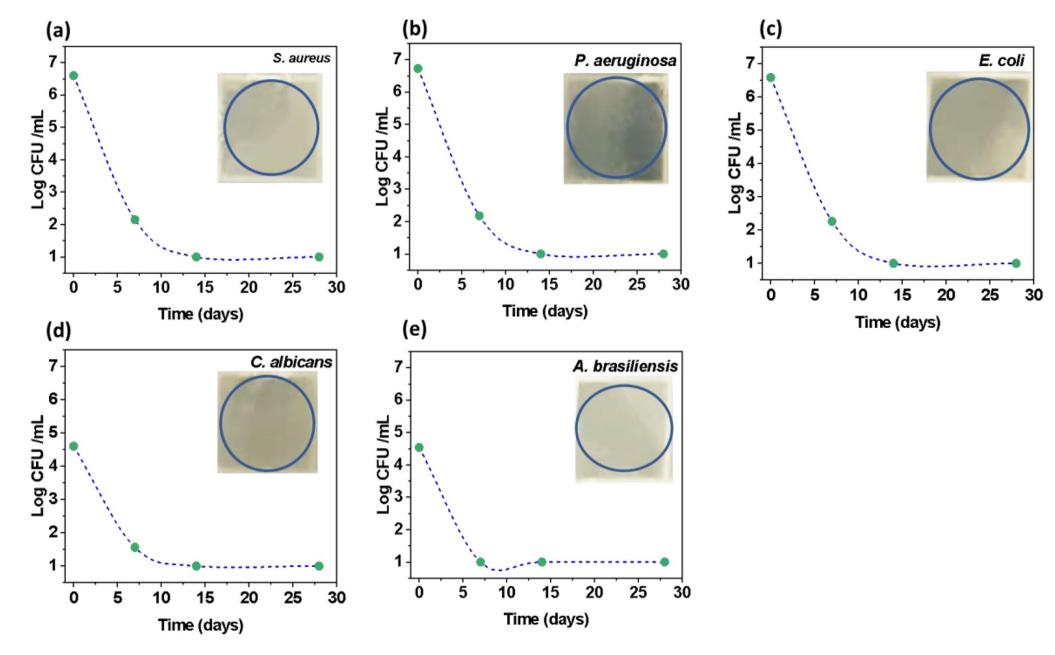 Fig.1 Biological efficacy of the synthesized PNCs against S. aureus, P. aeruginosa, E. coli, C. albicans, and A. brasiliensis organism with inset figures depicting the corresponding zone of the inhibition in a disc diffusion assay. (Ahmet Yıldız, et al. 2024)
Fig.1 Biological efficacy of the synthesized PNCs against S. aureus, P. aeruginosa, E. coli, C. albicans, and A. brasiliensis organism with inset figures depicting the corresponding zone of the inhibition in a disc diffusion assay. (Ahmet Yıldız, et al. 2024)
Nanoformulation biological evaluation includes the study of their metabolism, distribution and efficacy in the body. CD Formulation, as a world-class nanoformulation development and manufacturing service company, has rich experience in nanoformulation biological evaluation. If you are interested in nanoformulation biological evaluation, please feel free to contact us.
References
- Ahmet Yıldız, Tarık Küçükdeniz, Merve İlgar, et al. Integrated Data Modeling and Biological Evaluation of PEGylated Konjac Gum-Rosin Pentaerythritol Polymeric Nanocomposites for Enhanced Antimicrobial Performance. Journal of Polymers and the Environment. 2024, DOI: 10.1007/s10924-024-03270-0
How It Works
STEP 2
We'll email you to provide your quote and confirm order details if applicable.
STEP 3
Execute the project with real-time communication, and deliver the final report promptly.
Related Services



 Fig.1 Biological efficacy of the synthesized PNCs against S. aureus, P. aeruginosa, E. coli, C. albicans, and A. brasiliensis organism with inset figures depicting the corresponding zone of the inhibition in a disc diffusion assay. (Ahmet Yıldız, et al. 2024)
Fig.1 Biological efficacy of the synthesized PNCs against S. aureus, P. aeruginosa, E. coli, C. albicans, and A. brasiliensis organism with inset figures depicting the corresponding zone of the inhibition in a disc diffusion assay. (Ahmet Yıldız, et al. 2024)
