Nanoformulation Biodistribution Studies
Inquiry
The in vivo distribution of nanoformulations is intricately linked to their shape, size, surface characteristics, and properties. The targeted distribution of these formulations offers distinct benefits in facilitating drug release at specific sites and minimizing toxicity. CD Formulation plays a crucial role in advancing the development and clinical utilization of nanoformulations through the assessment of their biodistribution pathways and investigation of associated methodologies.
Why is Nanoformulation Biodistribution Study Important?
We conduct nanoformulation biodistribution research by evaluating the distribution pathways of nanoformulations in the body, which is of great significance to the development and clinical application of nanoformulations.
- Understand the distribution characteristics of nanoformulations in different organs and tissues to provide guidance for targeted drug delivery strategies.
- Evaluate the biocompatibility and potential toxicity of nanoformulations.
- Optimize the administration method and dosage regimen of nanoformulations to improve the therapeutic effect.
- Provide scientific basis for preclinical and clinical trials of nanoformulations.
Biodistribution Pathways of Nanoformulations
After nanoformulations enter the body, their biodistribution directly affects the efficacy and safety of nanoformulations. Nanoformulation biodistribution is mainly through the following pathways.
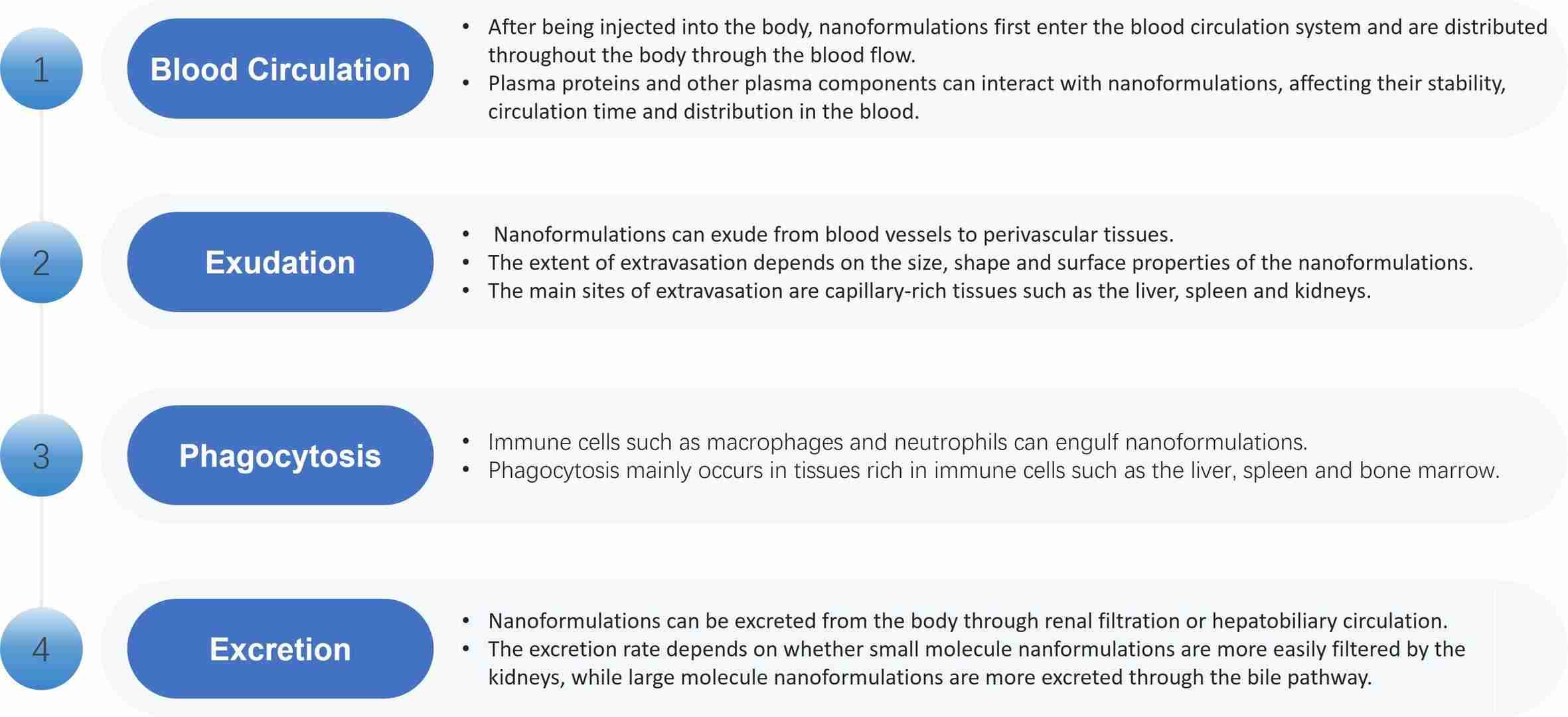 Fig.1 Biodistribution pathways of nanoformulations. (CD Formulation)
Fig.1 Biodistribution pathways of nanoformulations. (CD Formulation)
Our Services for Nanoformulation Biodistribution Studies
CD Formulation has achieved satisfactory results in nanoformulation biodistribution studies. We can provide researchers with nanoformulation biodistribution study services to aid the development and clinical application of nanoformulations.
Biodistribution Pathway Assessment Methods of Nanoformulations
Based on clinical expertise and experimental experience in nanoformulations, we explore a variety of methods for evaluating the nanoformulation biodistribution pathways, including but not limited to in vivo imaging, tissue distribution studies, pharmacokinetic studies, biodistribution modeling, and surface modification of nanoformulations.
In Vivo Imaging: In vivo imaging techniques, such as fluorescence imaging and photoacoustic imaging, can track the distribution of nanoformulations in the body in real time. We use this technology to provide dynamic information on the distribution of nanoformulations in different organs and tissues.
Tissue Distribution Study: After the animals are sacrificed, samples of different organs and tissues are collected and quantitative analysis of nanoformulations is performed. We use this method to provide cumulative distribution information of nanoformulations in different organs and tissues.
Pharmacokinetic Study: Pharmacokinetic studies include the concentration-time curve, clearance rate, and half-life of nanoformulations in the blood. Through this study, we can provide overall distribution and elimination information on nanoformulations in the body.
Biodistribution Modeling: Biodistribution modeling is a mathematical method used to predict the distribution and metabolism of nanoformulations in the body. The modeling we established can provide the distribution dynamics and distribution characteristics of nanoformulations in different tissues and organs.
Surface Modification of Nanoformulations: By modifying the surface of nanoformulations, we can change their distribution pathways in the body. For example, lipophilic modification can enhance the stability of nanoformulations in the blood, while hydrophilic modification can promote the exudation of nanoformulations.
Optimization and Selection of Nanoformulation Biodistribution Study Methods
We can provide an in-depth understanding of the in vivo nanoformulation biodistribution by optimizing the nanoformulation biodistribution study methods, selecting appropriate technologies and combining various information, thus providing an important basis for the evaluation of their safety and efficacy.
- In Vivo Imaging as Nanoformulation Biodistribution Study Methods
In vivo imaging is a non-invasive imaging technique that can be used to monitor the nanoformulation biodistribution in animals in real time. Our in vivo imaging techniques for monitoring nanoformulation biodistribution include the following techniques.
Optical Imaging: We use near-infrared fluorescent dyes or quantum dots to label nanoformulations and perform imaging through an optical detection system.
Radionuclide Labeling: We label radionuclides onto nanoformulations and perform imaging through a radionuclide imager.
Magnetic Resonance Imaging (MRI): We label nanoformulations with magnetic resonance imaging contrast agents for magnetic resonance imaging.
- Tissue Acquisition and Bioanalysis Methods
Tissue acquisition and bioanalysis is an invasive method that requires the dissection of animals to collect specific organ and tissue samples for drug concentration testing. Our tissue acquisition and bioanalysis methods are as follows.
Tissue Homogenization: We homogenize the collected tissue samples, extract drug components, and perform quantitative analysis through HPLC, LC-MS and other methods.
Thin Layer Chromatography (TLC): We spot the tissue sample extract on the TLC plate, develop it with solvent, separate different drug components, and perform qualitative or quantitative analysis through ultraviolet or fluorescence detection.
Immunohistochemistry (IHC): We use antibodies to specifically bind drugs or nanocarriers and observe the distribution of drugs in tissues through tissue section color development.
- Optimization of Nanoformulation Biodistribution Study Methods
Optimization of nanoformulation biodistribution study methods is critical to ensure data accuracy and reliability.
Selection of Dose and Time Point: We select appropriate doses and time points based on the pharmacokinetic properties of the nanoformulations in order to detect drug concentrations in appropriate tissues and organs.
Image Acquisition and Processing: We obtain clear, high-contrast images by optimizing the parameters of the in vivo imaging system (e.g., exposure time, filter selection). For tissue acquisition and bioanalysis, standardize tissue sample processing and analysis methods to ensure comparability of results.
Data Analysis and Modeling: We use pharmacokinetic modeling software or other statistical methods to analyze distribution data to derive biodistribution parameters of nanoformulations (such as volume of distribution, and clearance).
- Selection of Nanoformulation Biodistribution Study Methods
The selection of appropriate methods for the nanoformulation distribution study depends on the specific study objectives and the nature of the nanoformulations. We use a combination of different methods to provide complementary information to comprehensively evaluate nanoformulation biodistribution.
Why Choose Us for Studying Nanoformulation Biodistribution?
- Our core technical team has extensive disciplinary expertise in nano pharmaceuticals, nano pharmacology, nano pharmacodynamics, and nano toxicokinetics, and also has extensive experience in nanoformulation biodistribution studies.
- We have explored and conducted appropriate methods for monitoring the nanoformulation biodistribution, such as in vivo imaging, tissue section analysis, and quantitative biodistribution studies.
- We are also good at the optimization and selection of nanoformulation biodistribution study methods and can quickly respond to customers' detailed requirements for nanoformulation biodistribution studies.
Published Data
Technology: Biodistribution of aPD-1 NPs in Orthotopic HNSCC Models.
Journal: ACS Applied Materials & Interfaces
IF: 9.5
Published: 2022
Results:
The authors developed programmed cell death-1 antibodies (aPD-1) nanoformulations and studied their biodistribution after intratumoral injection in an orthotopic mouse model of head and neck cancer. Biodistribution analysis showed that nanoformulated aPD-1 had significantly lower distribution to off-target organs. Both aPD-1 NPs and free aPD-1 produced significantly higher tumor and tumor-draining lymph node accumulation. Thus, nanoformulations of aPD-1 can alter its locoregional and systemic distribution after intratumoral administration.
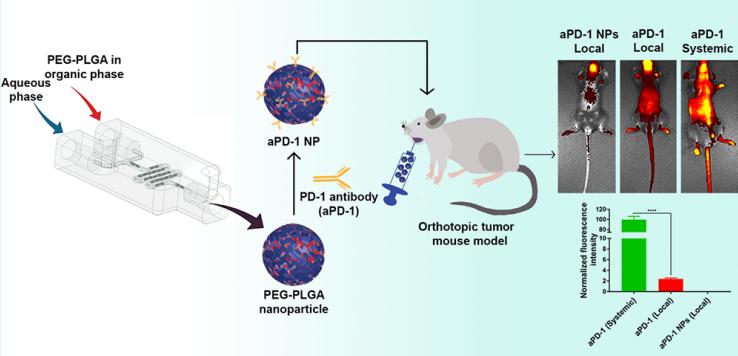 Fig.2 Biodistribution of aPD-1 NPs in Orthotopic HNSCC Models. (Parisa Badiee, et al. 2022)
Fig.2 Biodistribution of aPD-1 NPs in Orthotopic HNSCC Models. (Parisa Badiee, et al. 2022)
As the world's leading nanoformulation development and production services company, CD Formulation is devoted to researching nanoformulation formulations and is also committed to research on nanoformulation biodistribution. If you are interested in our nanoformulation biodistribution studies, please feel free to contact us.
References
- Parisa Badiee, Michelle F Maritz, Nicole Dmochowska, et al. Intratumoral Anti-PD-1 Nanoformulation Improves Its Biodistribution. ACS Applied Materials & Interfaces. 2022, 14(14):15881-15893.
How It Works
STEP 2
We'll email you to provide your quote and confirm order details if applicable.
STEP 3
Execute the project with real-time communication, and deliver the final report promptly.
Related Services


 Fig.1 Biodistribution pathways of nanoformulations. (CD Formulation)
Fig.1 Biodistribution pathways of nanoformulations. (CD Formulation) Fig.2 Biodistribution of aPD-1 NPs in Orthotopic HNSCC Models. (Parisa Badiee, et al. 2022)
Fig.2 Biodistribution of aPD-1 NPs in Orthotopic HNSCC Models. (Parisa Badiee, et al. 2022)
