Nanoformulation Non-clinical Safety Assessment
Inquiry
The primary objective of nonclinical safety assessment in nanoformulation development is to mitigate safety concerns in clinical trials. CD Formulation is dedicated to conducting nonclinical safety studies for nanoformulations to offer valuable insights for guiding clinical trials. This includes determining the appropriate dosage levels for clinical studies, particularly the initial dose in Phase Ⅰ trials, establishing safety monitoring parameters and schedules, assessing potential risks, and devising appropriate treatment strategies.
Why Conduct Nanoformulation Non-clinical Safety Assessment?
Nanoformulations exhibit unique biological characteristics as a result of their distinct physical and chemical properties, including nanoscale and nanostructure effects. These formulations possess the ability to selectively target specific organs, tissues, cells, and intracellular structures within the body through various mechanisms such as passive targeting, active targeting, physical targeting, and chemical targeting. This targeted delivery alters the pharmacokinetic profile of the drug, influencing its tissue distribution and ultimately impacting its safety and efficacy.
The unique characteristics of nanoformulations necessitate a thorough toxicological assessment to gather comprehensive non-clinical safety data. This approach allows for a comprehensive evaluation of potential risks associated with nanoformulations, providing valuable insights for the design of clinical trials and the judicious use of nanoformulations in clinical settings.
Our Solution for Nanoformulation Non-clinical Safety Assessment
CD Formulation attaches great importance to the non-clinical safety evaluation of nanoformulations and has a professional knowledge background and practical skills in the selection of testing systems, selection of test substances, and trial design for nanoformulation non-clinical safety evaluation. We can provide personalized professional technical support services to researchers around the world in preclinical research on nanoformulations.
 Fig.1 Our solution for nanoformulation non-clinical safety assessment. (CD Formulation)
Fig.1 Our solution for nanoformulation non-clinical safety assessment. (CD Formulation)
Test System Selection
In the nanoformulation non-clinical safety research, in order to obtain scientific and valid test data, a suitable test system should be selected.
Test Substance Selection
The test substance should be able to fully represent the sample intended for clinical use.
Trial Design for Nanoformulation Non-clinical Safety Assessment
Administration Dosage: When describing the dose-response relationship of nanoformulations, in addition to the traditional mass concentration, it is possible to consider providing the dosage unit information of mass concentration and number of nanoparticles/specific surface area at the same time.
Reference Group Setting: For nanoformulations containing novel active pharmaceutical ingredients, it is recommended to design a separate active ingredient group to examine the difference in safety between nanoformulations and separate active ingredients. For nanoformulations containing new nanocarriers, a separate drug-free nanocarrier group should generally be designed to examine the safety of new nanocarriers and their impact on the safety of active drug ingredients.
Detection Time and Frequency: Some nanoformulations are cleared slowly in tissues and may still accumulate even after stopping the drug for a period of time. The detection time point and detection frequency of toxicity indicators should be reasonably set according to the accumulation of nanoformulations in different tissues and organs. If necessary, it is possible to consider appropriately extending the recovery period and/or setting multiple recovery period observation time points.
Result Analysis and Risk Assessment: When conducting result analysis and risk assessment, we need to pay close attention to the nervous system, reproductive system and respiratory system toxicity, genotoxicity, carcinogenicity, immunogenicity, immunotoxicity, etc. related to nanoformulations and their active ingredients and/or carrier materials, and conduct a comprehensive analysis and assessment of tissue targeting, toxicity characteristics and mechanism of action.
Our Nanoformulation Non-clinical Safety Assessment Content
Nanoformulation non-clinical safety evaluation mainly includes safety pharmacology (general pharmacology), single-dose toxicity (acute toxicity), repeated-dose toxicity (long-term toxicity), genotoxicity, reproductive toxicity, carcinogenicity, dependence, special toxicity (allergic, local irritation, hemolysis), etc. CD Formulation is committed to studying the nanoformulation non-clinical safety assessment depending on our innovative and advanced design concept and professional experience.
Immunogenicity and Immunotoxicity
In the process of nanoformulation development and use, attention should be paid to the immunogenicity and immunotoxicity risks caused by nanoformulations due to their special properties, target conditions, proposed indications, immune status and history of the intended clinical population, route of administration, dosage, frequency and other related factors.
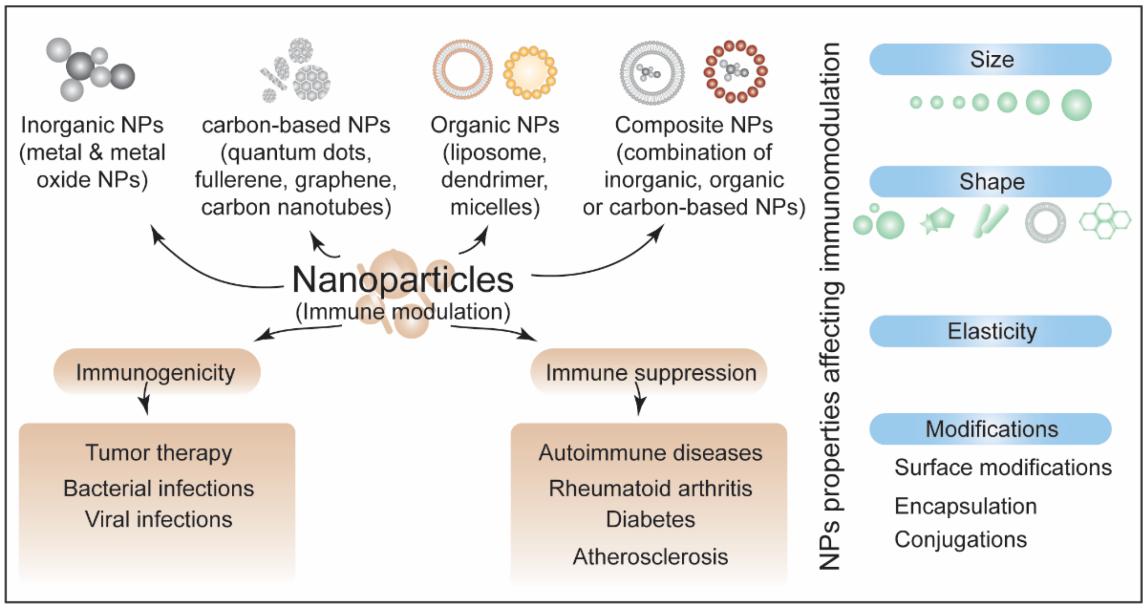 Fig.2 A schematic summarizing different forms of nanoparticles (NPs), aspects of immunomodulation, and the factors affecting these responses. (Ashutosh Pandey, et al. 2022)
Fig.2 A schematic summarizing different forms of nanoparticles (NPs), aspects of immunomodulation, and the factors affecting these responses. (Ashutosh Pandey, et al. 2022)
Nervous System Toxicity
Some nanoformulations enter the central nervous system after passing through the blood-brain barrier, producing corresponding biological effects and/or causing neurotoxicity. Therefore, for nanoformulations, attention should be paid to the situation of nanoformulations passing through the blood-brain barrier (such as blood-brain concentration ratio) to evaluate their potential neurotoxic effects.
Genotoxicity
Nanoformulations and new nanocarriers/excipients containing new active pharmaceutical ingredients need to undergo genotoxicity evaluation.
Carcinogenicity
The purpose of carcinogenicity studies is to identify potential carcinogenic effects in animals so that the associated risks in humans can be evaluated. Any concerns arising from laboratory studies, animal toxicology studies, and human data may lead to the need for carcinogenicity studies.
Reproductive Toxicity
Nanoformulations may easily pass through biological barriers such as the placental barrier, blood-testis barrier, and blood-milk barrier, thereby causing adverse effects on reproductive organs, fertility, embryo-fetal development, and offspring development. Therefore, attention should be paid to the reproductive toxicity risk of nanoformulations. Therefore, we need to pay attention to the distribution and accumulation of nanoformulations in reproductive organs.
Nanoformulation Safety
For injectable dosage forms, when conducting in vitro hemolysis tests, attention should be paid to whether nanoformulations will agglomerate in solution.
Toxicokinetics
Some nanoformulations may remain in tissues for a long time, and the tissue exposure may be higher than the systemic exposure. In particular, the retention time in tissues at toxic doses may be significantly longer than that at effective doses, and they may accumulate in certain tissues and organs in the body. This accumulation may produce obvious toxic reactions after multiple administrations of nanoformulations. Therefore, we should study the exposure, tissue distribution, and clearance (if necessary) of nanoformulations in systemic and/or local tissues, as well as the potential accumulation risk, through toxicokinetics to provide supporting data for the interpretation of the toxic characteristics of nanoformulations.
Why Choose Us for Nanoformulation Non-clinical Safety Assessment?
- We have accumulated rich experience in the nanoformulation non-clinical safety assessment including nanoformulation safety, toxicokinetics, immunogenicity and immunotoxicity, genotoxicity, etc.
- Our technical team has extensive expertise in nanoformulation non-clinical safety assessment. We are able to support you with nanoformulation non-clinical safety assessment during your nanoformulation development and before entering the clinical trial phase.
- We can provide you with our professional and personal nanoformulation non-clinical safety assessment trail design proposal according to your detailed requirements.
Published Data
Technology: Pre-clinical evaluation of A549 xenograft model
Journal: Colloids and Surfaces B: Biointerfaces
IF: 5.8
Published: 2023
Results:
The authors introduce rationally designed curcumin (CRC)-loaded exfoliated layered double hydroxide nanoparticles (X-LDH/CRC-NP) with a size of approximately 100 nm in non-small cell lung cancer (NSCLC) cell lines (A549 and NCI- H460) tested its suitability as a nanomedicine. The results showed that it enhanced apoptosis. Preclinical evaluation on the A549 tumor-bearing nude mouse model confirmed that these carefully designed exfoliated layered double hydroxide nanoparticles (X-LDH/CRC-NP) are very beneficial for the treatment of lung cancer.
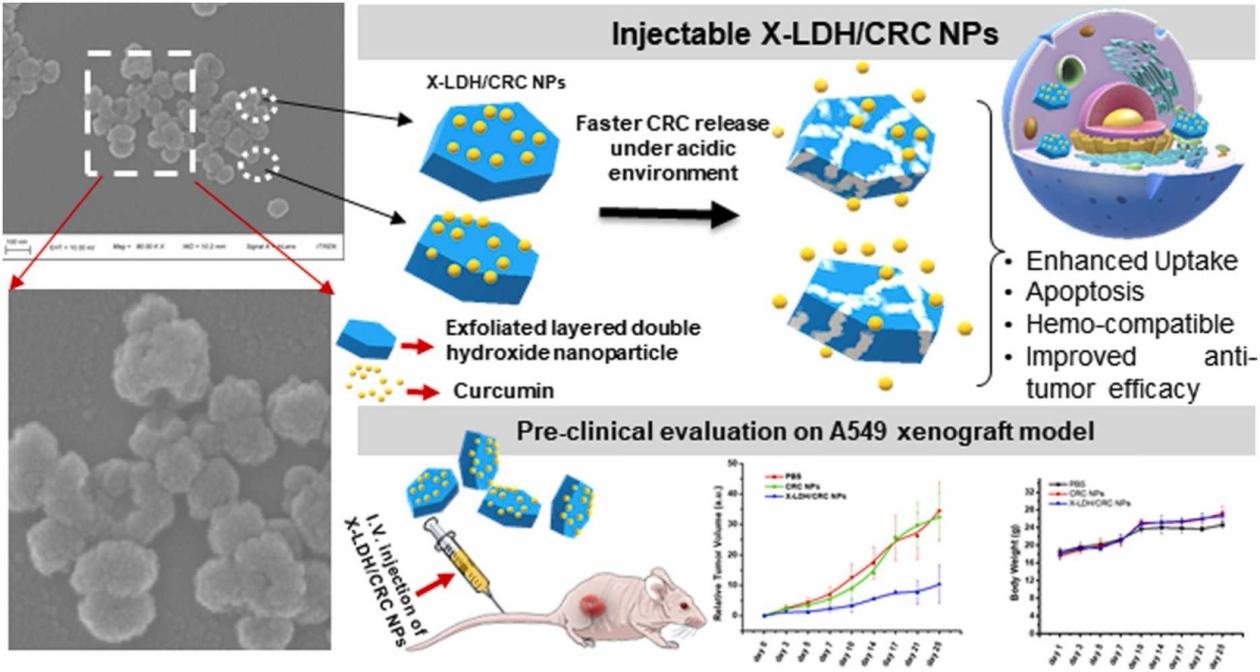 Fig.3 Pre-clinical evaluation of A549 xenograft model. (Sanoj Rejinold N, et al. 2023)
Fig.3 Pre-clinical evaluation of A549 xenograft model. (Sanoj Rejinold N, et al. 2023)
Nanoformulation non-clinical safety studies provide important reference information for the design of clinical trials of nanoformulations and rational clinical use of drugs. As a professional services company for the development of nanoformulations, CD Formulation has extensive experience in the non-clinical safety evaluation of nanoformulations and can provide customers with the necessary support in the design of clinical trials and rational clinical use of nanoformulations. If you require our nanoformulation non-clinical safety assessment services, please kindly contact us for in-depth discussions.
References
- Ashutosh Pandey, Abhinava K. Mishra. Immunomodulation, Toxicity, and Therapeutic Potential of Nanoparticles. BioTech. 2022, 11, 42.
- Sanoj Rejinold N, Huiyan Piao, Goeun Choi, et al. Curcumin in exfoliated layered double hydroxide nanoparticles: Pre-clinical evaluation as lung cancer nanomedicine. Colloids and Surfaces B: Biointerfaces. 2023, 228:113386.
How It Works
STEP 2
We'll email you to provide your quote and confirm order details if applicable.
STEP 3
Execute the project with real-time communication, and deliver the final report promptly.
Related Services


 Fig.1 Our solution for nanoformulation non-clinical safety assessment. (CD Formulation)
Fig.1 Our solution for nanoformulation non-clinical safety assessment. (CD Formulation) Fig.2 A schematic summarizing different forms of nanoparticles (NPs), aspects of immunomodulation, and the factors affecting these responses. (Ashutosh Pandey, et al. 2022)
Fig.2 A schematic summarizing different forms of nanoparticles (NPs), aspects of immunomodulation, and the factors affecting these responses. (Ashutosh Pandey, et al. 2022) Fig.3 Pre-clinical evaluation of A549 xenograft model. (Sanoj Rejinold N, et al. 2023)
Fig.3 Pre-clinical evaluation of A549 xenograft model. (Sanoj Rejinold N, et al. 2023)
