Nanoformulation Hemolysis Testing Service
Inquiry
Nanoformulation hemolytic activity is the hemolysis and red blood cell aggregation reactions caused by nanoformulations. The hemolytic activity test of nanoformulations is to observe whether the test substance can cause reactions such as hemolysis and red blood cell aggregation. CD Formulation has explored and developed multiple hemolysis testing methods of nanoparticles, including ultraviolet spectrophotometry, direct red blood cell counting method, liposome hemolysis method, etc.
Types of Hemolysis
Nanoformulation hemolysis is the rupture or destruction of red blood cells (RBCs), resulting in the release of hemoglobin and other cellular contents into the surrounding environment. It can occur in various situations and can be divided into three major categories: intravascular hemolysis, extravascular hemolysis, and immune-mediated hemolysis.
Intravascular Hemolysis
Intravascular hemolysis occurs within the blood vessels and involves the direct destruction of red blood cells. It can be caused by a variety of factors, including mechanical trauma, complement activation, infection, toxins, and incompatible blood transfusions. The release of hemoglobin and other cellular contents into the bloodstream during intravascular hemolysis can lead to various clinical manifestations, including hemoglobinemia, hemoglobinuria, and jaundice.
Extravascular Hemolysis
Extravascular hemolysis occurs outside the blood vessels, mainly in the spleen and liver. In this process, old or damaged red blood cells are recognized by macrophages and engulfed by them. The spleen is the main organ involved in this clearance process. Extravascular hemolysis is the most common type of hemolysis and occurs in various situations, including genetic diseases, autoimmune diseases, and certain infections. During extravascular hemolysis, engulfed RBCs are broken down and their components (including iron) are recycled for the production of new RBCs.
Immune-mediated Hemolysis
Immune-mediated hemolysis occurs when RBCs are targeted and destroyed by the immune system. It can be caused by autoantibodies (antibodies produced by the body against its own RBCs) or alloantibodies (antibodies produced against foreign RBCs, such as during a transfusion or during an Rh-incompatible pregnancy). The immune system recognizes the RBCs as foreign or abnormal and activates mechanisms to destroy them. Immune-mediated hemolysis can occur through both intravascular and extravascular mechanisms.
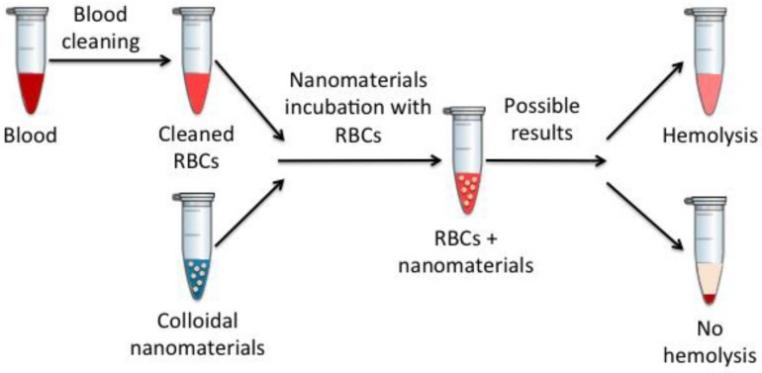 Fig.1 Schematic representation of the hemolysis assay. (Cristina Fornaguera, et al. 2017)
Fig.1 Schematic representation of the hemolysis assay. (Cristina Fornaguera, et al. 2017)
Why is Nanoformulation Hemolysis Testing Important?
The purpose of the nanoformulation hemolysis testing is to determine the impact of drugs or related preparations on the activities of red blood cells, complement systems, coagulation factors, and various enzymes in the blood by observing whether hemolysis occurs and its extent. The causes of hemolysis mainly include mechanical physical damage and soluble residual low-molecular chemical effects of materials. Hemolysis is divided into immune hemolysis and non-immune hemolysis according to whether there is an antigen-antibody reaction.
Immune hemolysis is the binding of related antigens to corresponding antibodies on the surface of red blood cells, causing hemolysis of red blood cells.
Non-immune hemolysis is caused by various physical and chemical factors, such as the irritation of drugs, the shape of related components in implant extracts, and the acidity and alkalinity of drugs.
Nanoformulation hemolysis testing is a very meaningful screening test in hematology and an important part of the safety evaluation of nanoformulations before they enter the market. Therefore, for nanoformulations, nanoformulations for injection, nanomedicine extracts, etc. that may cause various hemolytic reactions in clinical use, hemolysis evaluation is required.
Our Nanoformulation Hemolysis Testing Service
Hemolysis of nanoformulations refers to damage to red blood cells (RBCs), resulting in the release of the iron-containing protein hemoglobin into the plasma. CD Formulation has developed an in vitro hemolysis assay to analyze the hemolytic properties of nanoparticles.
In the nanoparticle hemolysis assay, analyte nanoparticles are incubated in blood, and hemoglobin is released from damaged cells and converted to red methemoglobin by a reagent. The nanoparticles and undamaged red blood cells (RBCs) are then removed by centrifugation, and the amount of methemoglobin in the supernatant is measured spectrophotometrically. Then the measured absorbance is compared to a standard curve to determine the hemoglobin concentration in the supernatant. Finally, this hemoglobin concentration is compared to the hemoglobin concentration in the supernatant of blood samples treated with negative control to obtain the percentage of nanoparticle-induced hemolysis. Here is the nanoparticle hemolysis testing process.
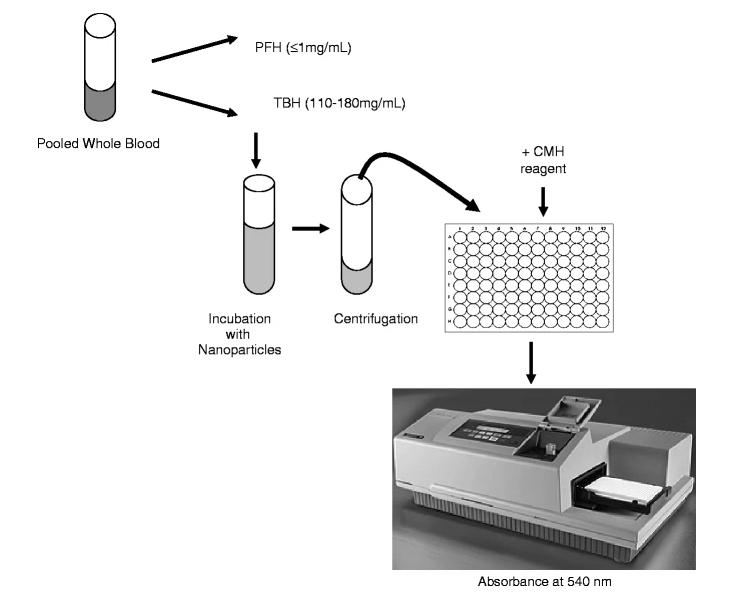 Fig.2 Workflow of nanoparticle hemolysis testing. (Barry W. Neun, et al. 2010)
Fig.2 Workflow of nanoparticle hemolysis testing. (Barry W. Neun, et al. 2010)
Explore Our Nanoformulation Hemolysis Testing Methods
After years of exploration and practice, we have summarized a variety of nanoformulation hemolysis detection methods. Based on detection indicators such as the number of red blood cells, enzymes released by rupture of red blood cells, and hemoglobin, we classify the nanoformulation hemolysis testing into the following categories.
Classification by Degree of Red Blood Cell Destruction
The main experimental methods for detecting hemolysis using this phenomenon include conventional in vitro hemolysis tests, direct red blood cell counting methods, and ultraviolet spectrophotometry.
Conventional in vitro hemolysis test is a test that uses whether red blood cells are lysed or not as an indicator of the occurrence of reaction.
The direct red blood cell counting method is to calculate the hemolysis rate by comparing the number of red blood cells before and after the test using a smear microscopy test result solution.
Ultraviolet spectrophotometry is based on the different absorption of drug molecules to electromagnetic waves of different wavelengths. It is a method of quantitative, qualitative and structural analysis.
Classification by Hemoglobin
This method reflects the hemolysis rate of drugs by detecting hemoglobin. Currently, the commonly used hemolysis test methods include 2,4-dichlorophenol method, ferricyanide method and benzidine method.
2,4-dichlorophenol method uses the principle that hemoglobin can catalyze the reaction of phenol, 4-aminoantipyrine and hydrogen peroxide to develop color to detect the hemolysis rate of nanoformulations.
Ferricyanide method generates a new detection index through the reaction between hemoglobin and potassium ferrocyanide, which indirectly reflects drug hemolysis.
Tobenzidine method uses the characteristic that heme in red blood cell hemoglobin has a peroxidase-like activity to test the hemolysis rate of drugs.
Other Methods
Serum lactate dehydrogenase activity assay utilizes the huge gap between serum lactate dehydrogenase inside and outside red blood cells and continuously detects the serum lactate dehydrogenase released from ruptured cells through a certain method to reflect the hemolysis rate of red blood cells.
Liposome hemolysis method is designed by taking advantage of the fact that liposomes and cell membranes have similar hydrophilic and hydrophobic amphoteric properties, and that high concentrations of fluorescent dyes in liposomes tend to cause self-shielding and loss of fluorescence properties.
Colloidal gold immunochromatography is a method in which chloroauric acid reacts with a reducing agent to polymerize into gold particles with stable molecular weight and then becomes a stable colloidal state under the action of electrostatics to perform drug hemolysis detection.
Why Choose Us for Testing Nanoformulation Hemolysis?
- We are good at exploring and developing nanoformulation hemolysis testing, covering direct red blood cell counting method, ultraviolet spectrophotometry, 2,4-dichlorophenol method, ferricyanide method, benzidine method, etc.
- We have established nanoformulation hemolysis testing methods to evaluate nanoparticle hemocompatibility before they enter the market.
- Our technical team is composed of experts with multidisciplinary backgrounds in nanomaterials, pharmaceutical preparations, biology, clinical medicine, etc., and can quickly respond to your requirements for nanoformulation hemolysis testing services.
Published Data
Technology: Hemolytic activity assay technology of nanoformulations
Journal: Asian Journal of Pharmaceutical Sciences
IF: 10.2
Published: 2023
Results:
Dexamethasone-loaded galactosylated PLGA/Eudragit S100/pullulan nanocargoes (Dexa-GP/ES/Pu NCs) were prepared by the authors using a modified emulsion method combined with solvent evaporation coating technology. Furthermore, the authors tested the nanocargoes using in vitro, ex vivo techniques, and dextran sulfate sodium (DSS)-induced UC models, such as Drug release and kinetics, In vitro biocompatibility studies, etc. The prepared nanocargoes had the desired physicochemical properties, drug release, cellular uptake, and cell viability. The results showed that Dexa-GP/ES/Pu NCs are biocompatible nanocargoes that can deliver drugs to the inflamed colon with unique targeting properties and a long duration.
Here is the result of the hemolytic activity assay of the nanoformulations after 24h and 48h.
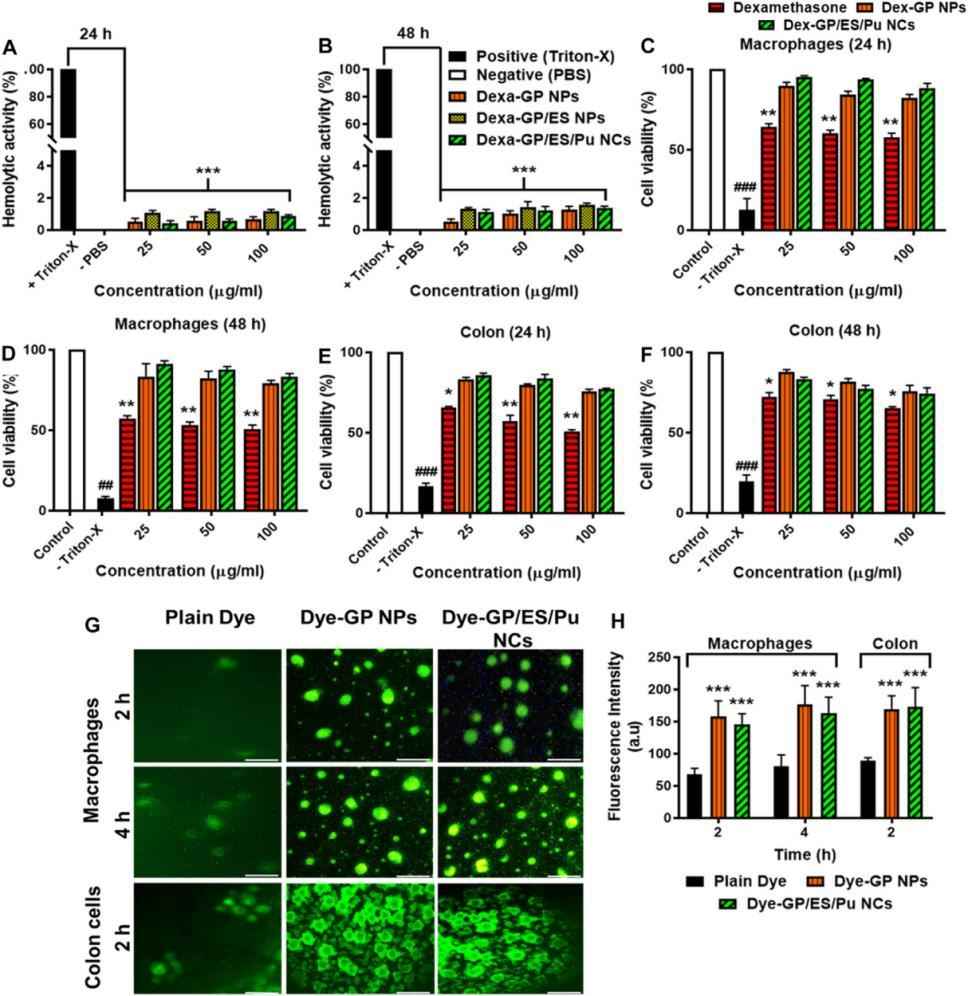 Fig.3 Hemolytic activity of nanoformulations. (Mahira Zeeshan, et al. 2023)
Fig.3 Hemolytic activity of nanoformulations. (Mahira Zeeshan, et al. 2023)
As your loyal partner in nanoformulation development and quality evaluation, CD Formulation has established a variety of nanoformulation hemolysis test methods to provide strong support for evaluating nanoformulation hemocompatibility. If you are interested in our nanoformulation hemolysis testing services, please contact us for detailed discussions.
References
- Cristina Fornaguera, Conxita Solans. Methods for the In Vitro Characterization of Nanomedicines—Biological Component Interaction. J Pers Med. 2017,7(1):2.
- Barry W. Neun, Marina A. Dobrovolskaia. Method for Analysis of Nanoparticle Hemolytic Properties In Vitro. Characterization of Nanoparticles Intended for Drug Delivery. 2010,697:215-224.
- Mahira Zeeshan, Qurat Ul Ain, Benno Weigmann, et al. Dual pH and microbial-sensitive galactosylated polymeric nanocargoes for multi-level targeting to combat ulcerative colitis. Asian Journal of Pharmaceutical Sciences. 2023,18(4):100831.
How It Works
STEP 2
We'll email you to provide your quote and confirm order details if applicable.
STEP 3
Execute the project with real-time communication, and deliver the final report promptly.
Related Services

 Fig.1 Schematic representation of the hemolysis assay. (Cristina Fornaguera, et al. 2017)
Fig.1 Schematic representation of the hemolysis assay. (Cristina Fornaguera, et al. 2017) Fig.2 Workflow of nanoparticle hemolysis testing. (Barry W. Neun, et al. 2010)
Fig.2 Workflow of nanoparticle hemolysis testing. (Barry W. Neun, et al. 2010) Fig.3 Hemolytic activity of nanoformulations. (Mahira Zeeshan, et al. 2023)
Fig.3 Hemolytic activity of nanoformulations. (Mahira Zeeshan, et al. 2023)
