Liposome Drug Efficacy Evaluation
Inquiry

CD Formulation is a global pharmaceutical company, and we have completed thousands of animal model preparation over the years, covering the digestive system, respiratory system, cardiovascular system, nervous system, tumor animal models to prepare more than 100 kinds of mature animal disease models. A complete set of pathology, histology, behavioral and other detection methods.
Why You Need Liposome Drug Efficacy Evaluation?
In the process of drug development, drug effectiveness evaluation is one of the keys to determine whether a drug can be marketed. Drug effectiveness studies include pharmacodynamics studies in preclinical animal trials and effectiveness studies in human clinical trials. Pharmacodynamic animal tests should be combined with the actual clinical diseases and treatment, reasonable selection of test models and methods, and design according to the classification of drugs and the characteristics of pharmacological action. For liposome drug development, animal pharmacodynamic tests are the basis of human clinical trials.
Explore Our Services for Liposome Drug Efficacy Evaluation
Our services for liposome drug efficacy evaluation are as following.
Screening of animal disease models
According to the mechanism of drug action, animal disease models with corresponding pathogenesis should be selected. If the mechanism of drug action is unclear, multiple models and methods should be used as far as possible to reflect and confirm the effectiveness of each other, and the experimental model should reflect the essence of clinical efficacy as far as possible.
Index screening.
This service mainly focus on the relationship between time and effect after drug administration
Liposome dose screening.
This service mainly aims to study on the relationship between dose and effect after drug administration.
Our Workflow of Liposome Drug Efficacy Evaluation
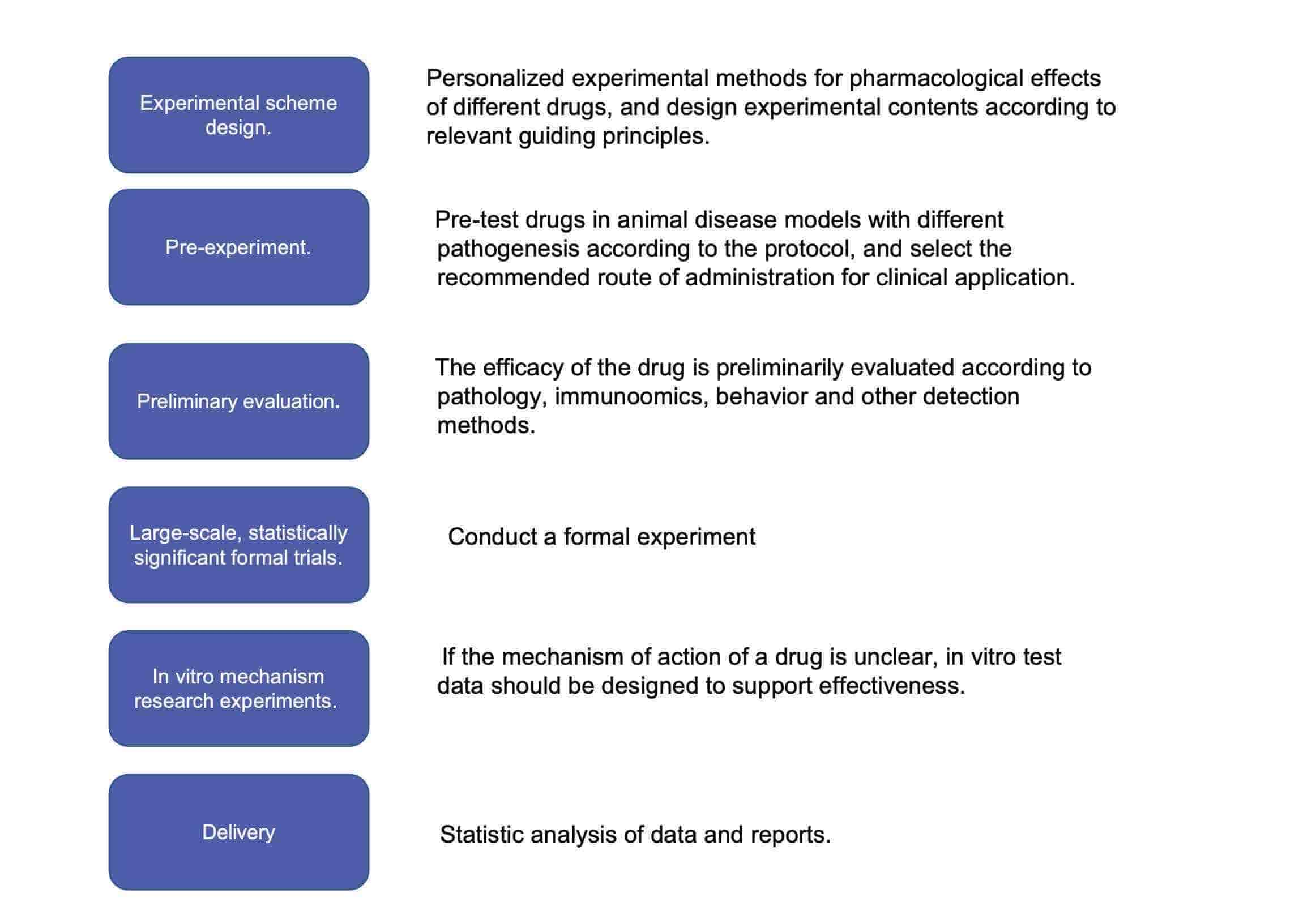 Fig.1 Our workflow for liposome drug efficacy evaluation. (CD Formulation)
Fig.1 Our workflow for liposome drug efficacy evaluation. (CD Formulation)
Our Platforms for Liposome Drug Efficacy Evaluation
| Platforms |
Specifics |
| Metabolic Disease Evaluation Platform |
- Model of non-alcoholic fatty liver in cynomolgus monkey(non-alcohliver disease, NAFLD)
- Liver fibrosis model of cynomolgus monkey
- Efficacy test of normal monkey glucose tolerance model
- STZ-induced diabetes model in cyborg monkeys or rhesus monkeys
- Obesity and diabetes rat model Nonalcoholic fatty liver model (NAFLD, NAFL, NASH)
- Osteoporosis model
- Renal failure model
- Dyslipidemia model
- Spontaneous or induced obesity in rodent models
|
| Evaluation of Immune System DiseasesPlatform |
- Monkey challenge test model of human vaccine
- Rheumatoid arthritis CIA mouse/rat model
- Rat model of acute joint inflammation induced by carrageenan
- EAE experimental allergic encephalomyelitis mouse model
- Myocardial tablet mouse ear transplantation model (to observe the inhibitory effect of drugs on host immune system)
- Canavalin protein A(ConA) induced mouse liver fibrosis model
- acute joint inflammation induced by carrageenan.
|
| Nervous System Evaluation Platform |
- The monkey depression model induced by activity space restriction
- Mouse model of Alzheimer's disease (behavioral test, spontaneous activity meter, rod rotator)
- Mouse model of Parkinson's disease (behavioral test, spontaneous motility tester, rod rotator)
- Pain models in rats and mice (hot plate instrument, electric stimulation instrument, etc.)
|
| Nervous System Evaluation Platform |
- Rat hypertension (SHR) model
- Thrombus model in vivo induced by collagen mixed with epinephrine injected into the tail vein of mice
- Rat tail artery thrombosis model induced by subcutaneous injection of carrageenan ferric chloride (FeCl3) induced common carotid artery and middle cerebral artery (MCA) thrombosis model.
|
| Ophthalmic Drug Evaluation platform |
- Efficacy model of vitreous administration: rabbit, dog, monkey; (vitreous administration, efficacy test: fundoscope, electroretinogram) dry eye in rats, mice, rabbits and other animals.
- Liver fibrosis model of cynomolgus monkey.
|
Highlights for Liposome Drug Efficacy Evaluation
- Specialty. We possess ISO 17025 accreditation, registration for BSL-2 (Biological Safety Level 2) laboratory, license for the utilization of experimental animals, radiation safety license, and registration for radioactive substances, which guarantees liposome drug efficacy evaluation services.
- Empirical. The laboratory staff members possess extensive years of experience in conducting in vivo pharmacological researches including liposome drug efficacy evaluation.
- Timely. Communicate thoroughly with the customer prior to project commencement, promptly respond, and actively explore potential partnerships to advance the projects of liposome drug efficacy evaluation.
Published Data
Technology: culture-based microtitration method in liposomal formulation of amphotericin B
Journal: Indian J Med Res
IF: 4.2
Published: 2013
Results: In this study, the author evaluated the in vivo anti-leishmanial efficacy and related side effects of Kalsome™10 in BALB/c mice. The traditional method for detecting and quantifying the parasite burden in different mouse tissues and evaluating the anti-leishmanial efficacy of the drug, which involves microscopic counting of the amastigote forms in the host cell nucleus, is still used. This method is time-consuming and subjective, and may not be reliable if the parasites are not evenly distributed on the slide. Therefore, a culture-based microtitration method was developed. This method is more sensitive than the smear method, but labor-intensive and time-consuming. Other limitations include the very low number of parasites. Since the recurrence of leishmaniosis is related to the tissue burden of residual and dormant parasites after treatment, quantitative PCR (qPCR) detection is not useful for indicating positive or negative results. Therefore, in this study, the efficacy of the novel anti-leishmanial agent was evaluated by quantitative real-time PCR (qRT-PCR) using primers targeting the conserved region of the internal transcribed spacer (ITS) of the DNA of the Duonowanilishmanial nDNA as evidence of its anti-leishmanial activity.
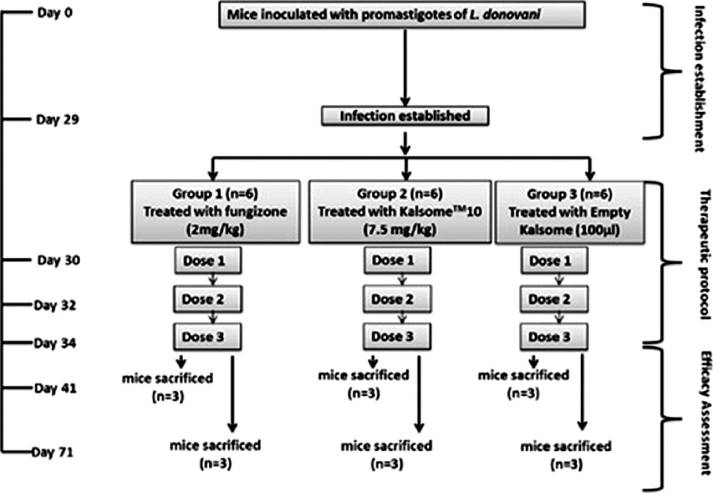 Fig.2 Flowchart summarizing the experimental details of the study. (Mishra J, et al., 2013)
Fig.2 Flowchart summarizing the experimental details of the study. (Mishra J, et al., 2013)
CD Formulation has accumulated many years of project experience in the study of the drug efficacy evaluation, and our team of scientists and experts are ready to answer your questions and provide advice. If you need our help, please contact us at your convenience.
References
- Mishra J, Dey A, et al. Evaluation of toxicity & therapeutic efficacy of a new liposomal formulation of amphotericin B in a mouse model. Indian J Med Res. 2013 Apr;137(4):767-76
How It Works
STEP 2
We'll email you to provide your quote and confirm order details if applicable.
STEP 3
Execute the project with real-time communication, and deliver the final report promptly.
Related Services



 Fig.1 Our workflow for liposome drug efficacy evaluation. (CD Formulation)
Fig.1 Our workflow for liposome drug efficacy evaluation. (CD Formulation) Fig.2 Flowchart summarizing the experimental details of the study. (Mishra J, et al., 2013)
Fig.2 Flowchart summarizing the experimental details of the study. (Mishra J, et al., 2013)
