Liposome In Vitro Bioactivity Evaluation
Inquiry

The biological activity method is the only in vitro analysis method that reflects the clinical effect of liposome drugs. In terms of in vitro bioactivity evaluation of liposomes, CD Formulation has the most advanced in vitro evaluation instruments and concepts, equipped with a variety of dissolution & release equipment, penetration equipment, transdermal equipment, and established a variety of in vitro evaluation models for different dosage forms such as oral liposome, topical liposomes, injection liposomes, etc. We are working with a number of pharmaceutical customers to continuously expand our capabilities in drug bioequivalence evaluation in vitro, provide customers with high-quality and efficient overall solutions and strategies, and continue to help the development and marketing of liposome drugs.
Why You Need Liposome In Vitro Bioactivity Evaluation?
In vitro bioactivity evaluation is the determination of the active ingredients and efficacy of liposome drugs, which is a very important part of drug quality control. Biological activity detection runs through the entire life cycle of liposome drugs, from early target discovery, antibody screening, transition to CMC stage for production, clinical application, and finally marketing, which will involve the analysis of biological activity. Therefore, it is extremely important to select an efficient, rapid and accurate method for the determination of biological activity.
Explore Our Services for Liposome In Vitro Bioactivity Evaluation
Liposomes are usually used as carriers to deliver drugs, but the actual active substance is the encapsulated active substance. Therefore, the in vitro activity of the main active substance should be paid attention to when evaluating the biological activity of liposomes. In addition, the mechanism of action of each bioactive substance is different, especially for targeted modified liposomes, we need to screen different in vitro activity evaluation strategies according to different targeted modifiers. Our services include but are not limits to the following.
Functional activity assay
- In vitro cytotoxicity assay. Cell proliferation inhibition method is used to evaluate the biological activity of drugs, mainly by detecting cell viability and reflecting cell proliferation or death after treatment with liposomes at different concentrations under different culture systems.
- Anticancer activity analysis. This service primarily assesses anticancer effects by examining the effective inhibition of liposomes on the proliferation of cancer cell lines
- Evaluation of anti-inflammatory effects. This service is evaluated primarily by examining the effectiveness of liposomal drugs in reducing the expression of pro-inflammatory cytokines/chemokines in cells.
Binding activity assay
- Evaluation of binding to targeted cells. This service aims to analysis the targeted modification of liposomes, e.g. immunoliposomes. According to different types of modifier, liposomes can be divided into peptides, antibodies, sugars, ligands, and aptamers.
- Receptor selective binding analysis. This service aims to Ligand-modified liposomes.
Our Workflow of Liposome In Vitro Bioactivity Evaluation
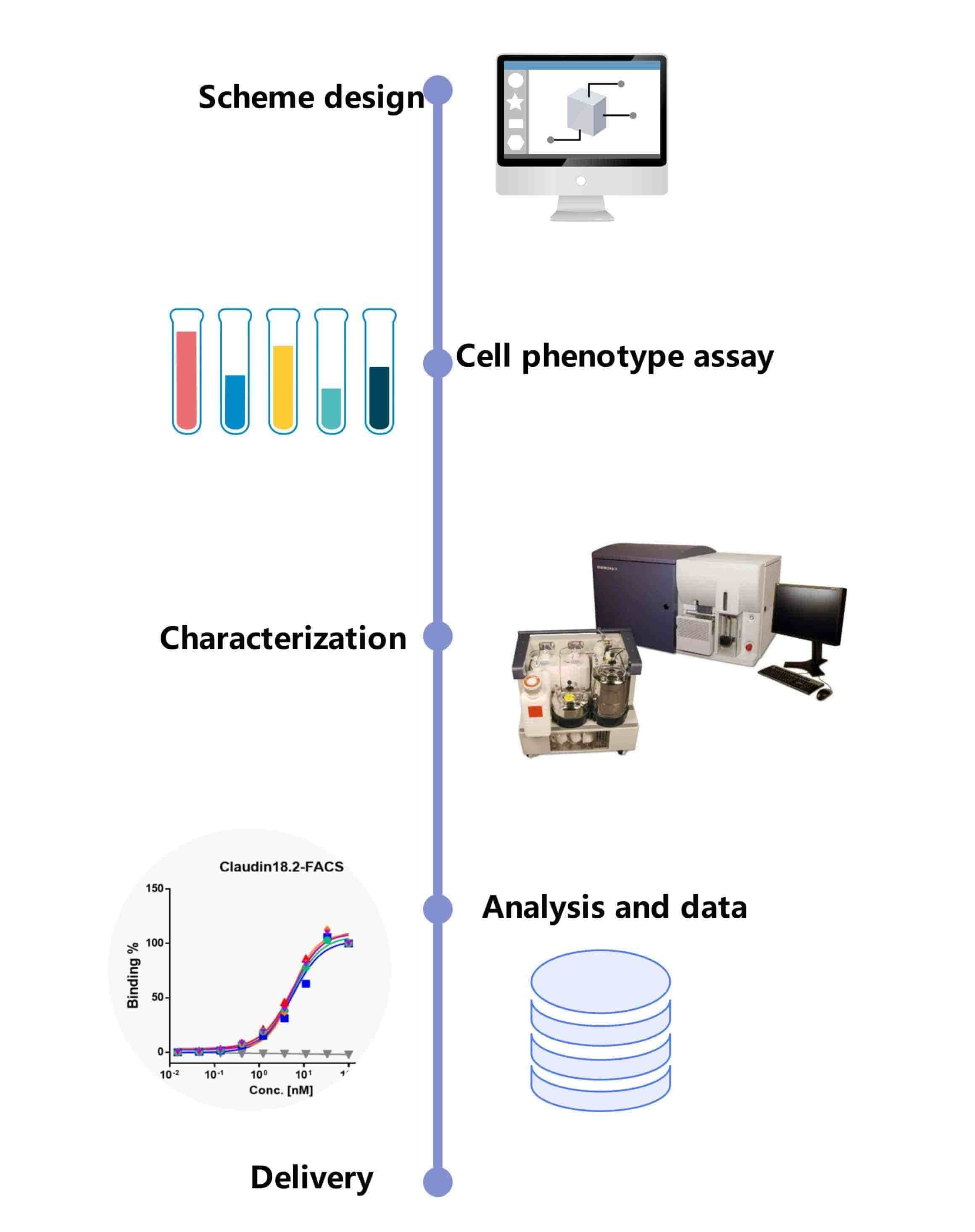 Fig.1 Our workflow for liposome in vitro bioactivity evaluation. (CD Formulation)
Fig.1 Our workflow for liposome in vitro bioactivity evaluation. (CD Formulation)
Our Platforms for Liposome In Vitro Bioactivity Evaluation
| Platforms |
Specifics |
| Cytological experimentPlatform |
We provide cytological experimental models of different types of targets, and hundreds of disease models have been developed.
- in vitro evaluation model of transdermal preparations
- parallel artificial membrane permeability assay (PAMPA)
- In vitro skin organoid model
|
| In vitro evaluation system |
- There are dozens of multi-dimensional and three-dimensional artificial efficacy evaluation systems, from cell sorting to induced differentiation, from flow cell screening to in vitro function screening, covering most applications, which can meet the evaluation needs of antigen and antibody binding, signaling pathway, cell function and other different levels.
|
Highlights for Liposome In Vitro Bioactivity Evaluation
- Strict. Strict quality management system from personnel operations to instruments to confirm the accuracy of liposome in vitro bioactivity evaluation results.
- Expertise. Our laboratory staff have an average of many years of experience in conducting in vivo bioactivity evaluation.
- Timely. Advanced project management mode developed liposome in vitro bioactivity evaluation services for ensures efficient and fast delivery of satisfactory experimental results to customers.
Published Data
Technology: shikotin liposomes technology
Journal: Phytomedicine
IF: 7.9
Published: 2022
Results: In this study, the authors developed shikotin liposomes, which showed good slow-release behavior and successfully improved the antibacterial efficiency of SH against Staphylococcus aureus. The mechanism of the designed SH-liposome's antibacterial activity against Staphylococcus aureus is related to the irreversible damage to the bacterial cell wall and membrane. In addition, SH-liposome preparations play a beneficial role in the treatment of MRSA-infected burn wounds. Overall, SH-liposome is a promising novel and effective.
For many years, CD has helped many customers study the in vitro bioactivity of liposomes, and our team of scientists and experts have the expertise and knowledge to answer your questions. If you are interested in our services, please contact us at your convenience.
References
- Gang Shu, Dan Xu, et al. Preparation of shikonin liposome and evaluation of its in vitro antibacterial and in vivo infected wound healing activity. Phytomedicine. 2022, 99, 154035.
How It Works
STEP 2
We'll email you to provide your quote and confirm order details if applicable.
STEP 3
Execute the project with real-time communication, and deliver the final report promptly.
Related Services



 Fig.1 Our workflow for liposome in vitro bioactivity evaluation. (CD Formulation)
Fig.1 Our workflow for liposome in vitro bioactivity evaluation. (CD Formulation)
