Liposome Pharmacokinetics Study
Inquiry
CD Formulation pharmacokinetics research platform was established in 2012. Relying on the perfect technical service platform for bioassay, data management and statistical analysis, we have grown into a global leader in the field of pharmacokinetics research services, accumulated rich project experience, and have a professional technical team, It can efficiently provide high-quality research results, meet the normative and personalized needs of partners for research and development projects, and is committed to becoming the most ideal pharmacokinetic research partner for drug research and development enterprises.
Why You Need Liposome Pharmacokinetics Study?
The pharmacokinetic study of liposome provides accurate data for preclinical trials and provides basis for related clinical trials. Not only can the initial dose be precisely measured, but potential side effects can also be controlled. Animal models are typically used before drugs are tested on humans. In addition, we also need to minimize the effects of the prescription and dosing method used on drug exposure and on the health of the animals used before the experiment. Through pharmacokinetic studies, it can also be more comprehensive and accurate to understand the changes of drug effects with dose and time, and obtain drug efficacy exposure, onset time and maintenance time, which can provide an important reference for the dose and efficacy interval of clinical trials.
Explore Our Services for Liposome Pharmacokinetics Study
At the study center of CD Formulation, we have complete in vitro ADME research, in vivo pharmacokinetics, metabolite identification and other related capabilities, and have excellent service capabilities and project experience. We can provide customized services to our partners, providing customized solutions according to the characteristics of the project at different stages of drug development.
In vitro ADME study
This service aims to help partners understand the absorption, distribution, metabolism, and excretion properties of drugs, evaluate potential drug interactions, and facilitate liposome formulation development.
- Absorption. In vitro permeability model was used to evaluate the permeability. Study of in vivo absorption in animal models. At this service, we offer solvent screening service. Polyethylene glycol (PEG) lipids are widely used as carrier materials in liposomes.
- Distribution. Tissue distribution studies or QWBA methods are used to investigate drug distribution.
- Metabolism. Whole blood/plasma stability study, liver and kidney stability study, metabolic product identification to explore metabolic transformation
- Excretion. Investigation of metabolic pathways in animals.
Evaluation of pharmacokinetics in vivo
This service aims to fully elucidate the characteristics of drug absorption, distribution, metabolism, and excretion in the body.
- Single dose pharmacokinetics and dose ratio study
- Multiple administration pharmacokinetics study
- Multi-cycle crossover BE study
- Mouse/rat/free/dog/miniature pig/crab-eating monkey
- Intravenous/oral/subcutaneous/percutaneous/muscular/abdominal/sublingual/nasal/pulmonary inhalation/vitreous administration
- Venous, arterial catheterization/biliary catheterization
- Cassette dosing/continuous microblood collection
- Tissue distribution/blood-brain barrier permeability
- Drug accumulation and targeting evaluation
- In vivo metabolite identification
- Drug-Drug Interaction.
PK Bioanalysis services
Through PK analysis of carrier materials, we help customers analyze and exclude the influence of carrier materials on the biological behavior and pharmacological action of liposomes in vivo.
Our Workflow for Liposome Pharmacokinetics Study
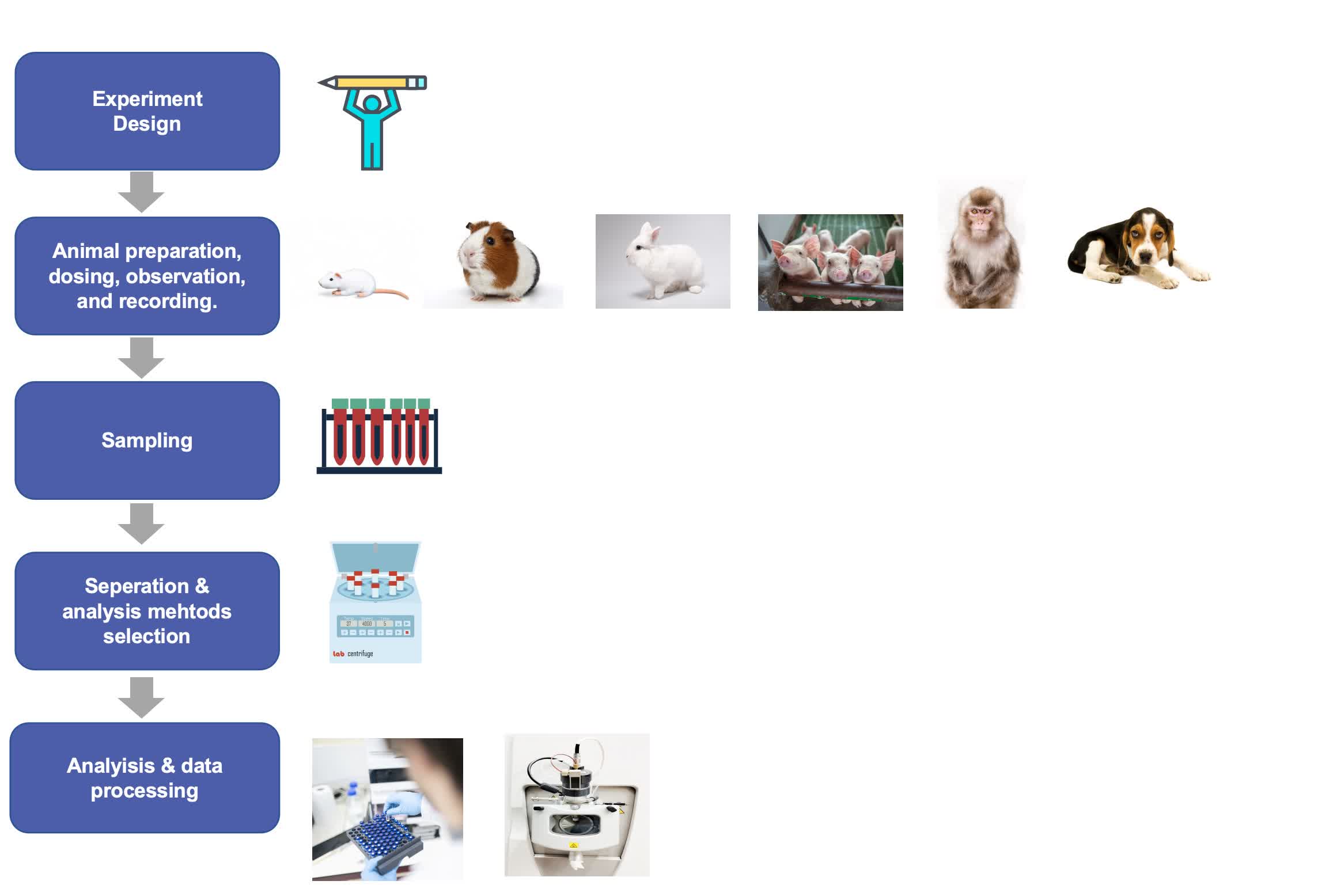 Fig.1 The workflow for liposome pharmacokinetics study. (CD Formulation)
Fig.1 The workflow for liposome pharmacokinetics study. (CD Formulation)
Our Platforms for Liposome Pharmacokinetics Study
| Equipments & Platforms |
Specifics |
Advanced Bioanalysis Platform
 |
Sciex Triple Quad 7500; Sciex Triple Quad 6500+; QTRAP 6500; Thermo Fisher Scientific Q Exactive HF-X ICP-MS ; Waters Q-TOF Thermo Scientific GC-MS Agilent GC-MS Fluorescent quantitative PCR instrument MSD; Simoa; Microplate System ELISPOT; Flow Cytometer Liquid scintillation counter solid scintillation counter Gamma counter; Oxidation burner, etc. |
Laboratory Animal Centre
 |
The laboratory animal center (including radioactive barrier and ordinary environment) covers an area of about 5500 m2 (including office area), with SPF and ordinary animal feeding facilities, and has set up the animal management and use committee of the center for non-clinical evaluation of drugs (IACUC) to strengthen the daily management of laboratory animals and animal tests in the center. |
Highlights for Liposome Pharmacokinetics Study
- High standard quality management system. ISO 17025 certification, Biosafety Level 2 (BSL-2) laboratory registration certificate, laboratory animal use license, radiation safety license, radioisotope registration certificate, etc.
- Mature project management system. We use Thermo Watson LIMS laboratory information management System 7.5 to ensure accurate management of the entire life cycle of test samples.
- Timely communication. We have fully communicated with each other about the progress of the project, responded quickly, and actively considered for our partners to jointly promote the project about liposome pharmacokinetics study.
Published Data
Technology: stealth liposomes technology
Journal: Topics in Catalysis
IF: 3.6
Published: 2023
Results: In this study, the authors developed a novel apigenin-loaded invisible liposome to improve its solubility, plasma retention time and better therapeutic effect. Invisible liposomes (APISL) loaded with apigenin were prepared by ethanol injection. The U.S. Department of Energy performed statistical optimization of the formulation and evaluated particle size, zeta potential, encapsulation efficiency, morphology, in vitro drug release studies, in vivo pharmacokinetic studies, and stability studies. According to the Higuchi release model, the optimized apigenin-loaded invisible liposome L8 showed a higher percentage of drug release than ordinary apigenin. According to in vivo experiments, the exposure time of invisible liposomes is longer than that of pure drug solutions. Compared to standard drugs, studies have shown that apigenin-loaded invisible liposomes exhibit better plasma retention time and stability. Therefore, the results suggest that apigenin-loaded stealth liposomes exhibit improved bioavailability and enhanced anti-tumor efficacy in breast cancer therapy.
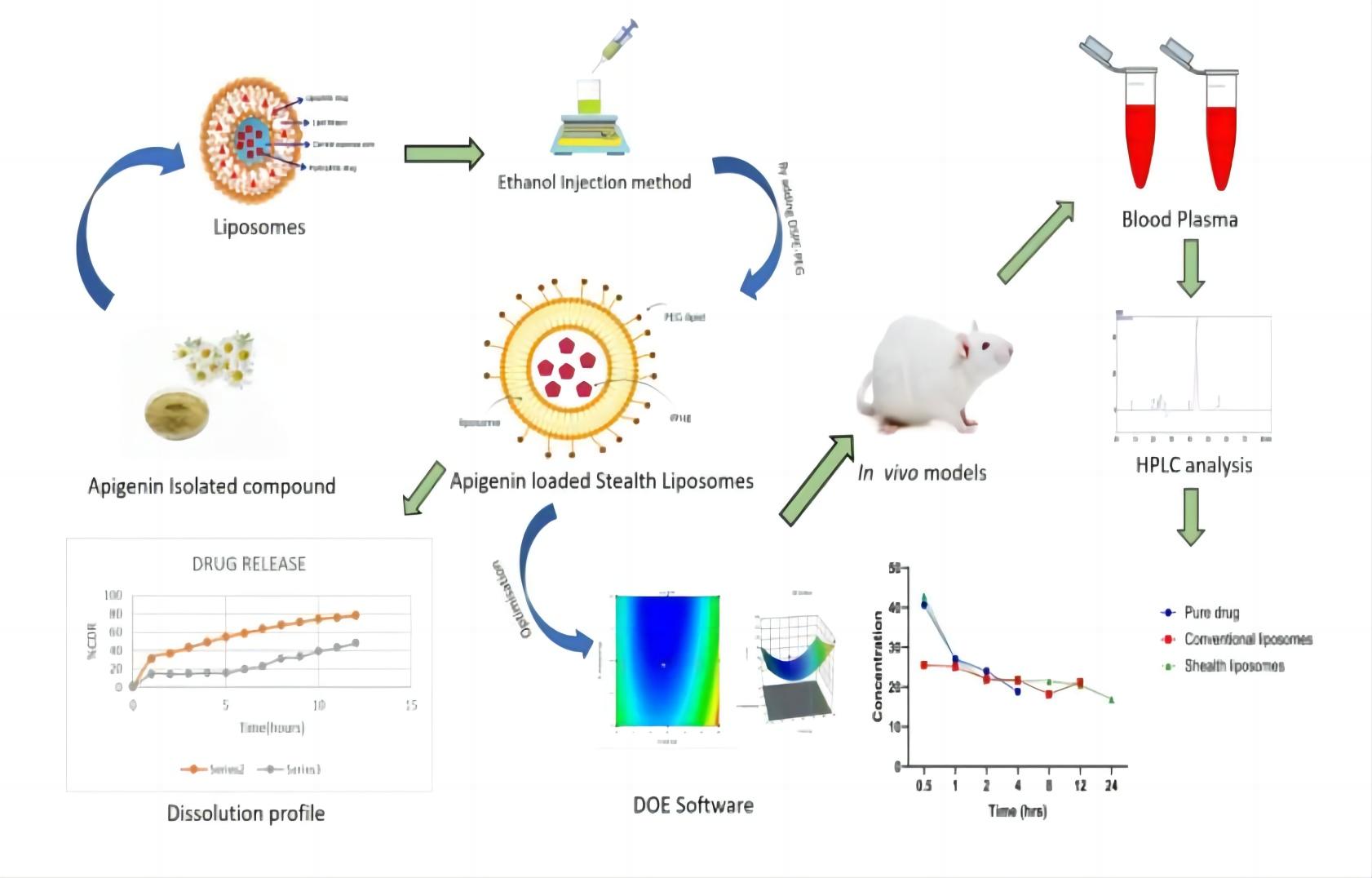 Fig.2 The process of apigenin-loaded stealth liposomes pharmacokinetic study. (Shetti, P., et al., 2023)
Fig.2 The process of apigenin-loaded stealth liposomes pharmacokinetic study. (Shetti, P., et al., 2023)
With extensive project experience and an advanced analytical platform, CD Formulation has extensive expertise in liposomal pharmacokinetic studies. Our team of scientists is on hand to address any queries and provide consulting services. If you need our help, please contact us by phone or email at your convenience.
References
- Shetti, P., Jalalpure, S.S., et al. Apigenin-Loaded Stealth Liposomes: Development and Pharmacokinetic Studies for Enhanced Plasma Retention of Drug in Cancer Therapy. Top Catal 2024, 67, 46–58.
How It Works
STEP 2
We'll email you to provide your quote and confirm order details if applicable.
STEP 3
Execute the project with real-time communication, and deliver the final report promptly.
Related Services


 Fig.1 The workflow for liposome pharmacokinetics study. (CD Formulation)
Fig.1 The workflow for liposome pharmacokinetics study. (CD Formulation)

 Fig.2 The process of apigenin-loaded stealth liposomes pharmacokinetic study. (Shetti, P., et al., 2023)
Fig.2 The process of apigenin-loaded stealth liposomes pharmacokinetic study. (Shetti, P., et al., 2023)
