Liposome In Vitro Release Study
Inquiry
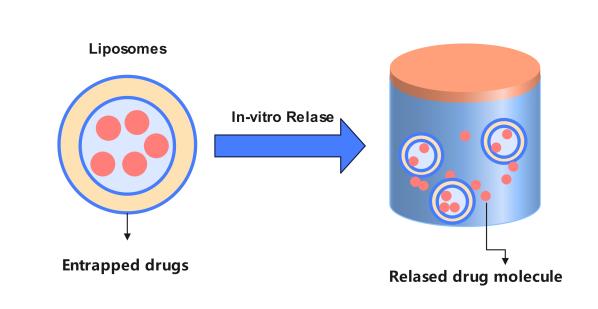
The quality control and evaluation of liposomal formulations are essential, with in vitro release studies playing a pivotal role in assessing their quality. As a leading global pharmaceutical technology company, dedicated liposome in vitro analysis team of CD Formulation strives to advance the development of liposomes in various fields by offering expert-led in vitro release studies for different types of liposomes through our cutting-edge platform.
Why You Need Liposome In Vitro Release Study?
In the ideal scenario, liposomes are transported to the target organ, tissue, or cell upon entering the human body. Subsequently, these active ingredients are released freely from the liposomes at the target site to exert their clinical effects. However, due to high costs and risks associated with clinical trials, assessing in vitro release can be utilized as a predictive tool for in vivo behavior and mitigate potential failures during clinical trials. In addition to reflecting physicochemical properties such as morphology, particle size and distribution, encapsulation rate, leakage rate of liposome drugs, in vitro release also differentiates significantly between batches prepared with varying prescriptions or compositions. This distinction provides insights into safety and effectiveness to some extent. Therefore, evaluating in vitro release is considered a crucial quality control parameter for liposomes.
Explore Our Services for Liposome In Vitro Release Study
With years of experience and specialized knowledge in the field, we are capable of assisting you in every step of the research and development process. Our services include, but are not limited to:
Study of In vitro Release Conditions
The focus of this service lies in the assessment of liposomal drug release/leakage under various conditions (e.g., pH, temperature, plasma). Throughout this process, careful consideration should be given to the liposomal drug's design objectives, administration method, and intended application. Each delivery route necessitates distinct release tests that closely simulate in vivo conditions.
Study on Separation Methods
The current separation methods we can provide for free drugs and liposome-encapsulated drugs are primarily categorized into three groups: dialysis-based separation; centrifugation-based separation; gel chromatography-based separation(such as size exclusion chromatography).
The Development and Validation of Evaluation Methods for Liposome In Vitro Release
The methods we can provide for evaluating the release behavior of liposomes are primarily categorized into sample separation methods, dialysis, flow cell methods, Franz diffusion cell methods, and ussing chamber methods. Different types of liposomal drugs require different testing approaches for release rates. However, especially with emerging or improved techniques, there is a lack of standardization, limited research verification, and narrow application scope which introduces uncertainty in quality control and evaluation. Therefore, establishing repeatable, user-friendly, and highly standardized release rate methodologies poses a significant technical challenge in the development of in vitro liposome release behavior.
General Workflow of Liposome In Vitro Release Study
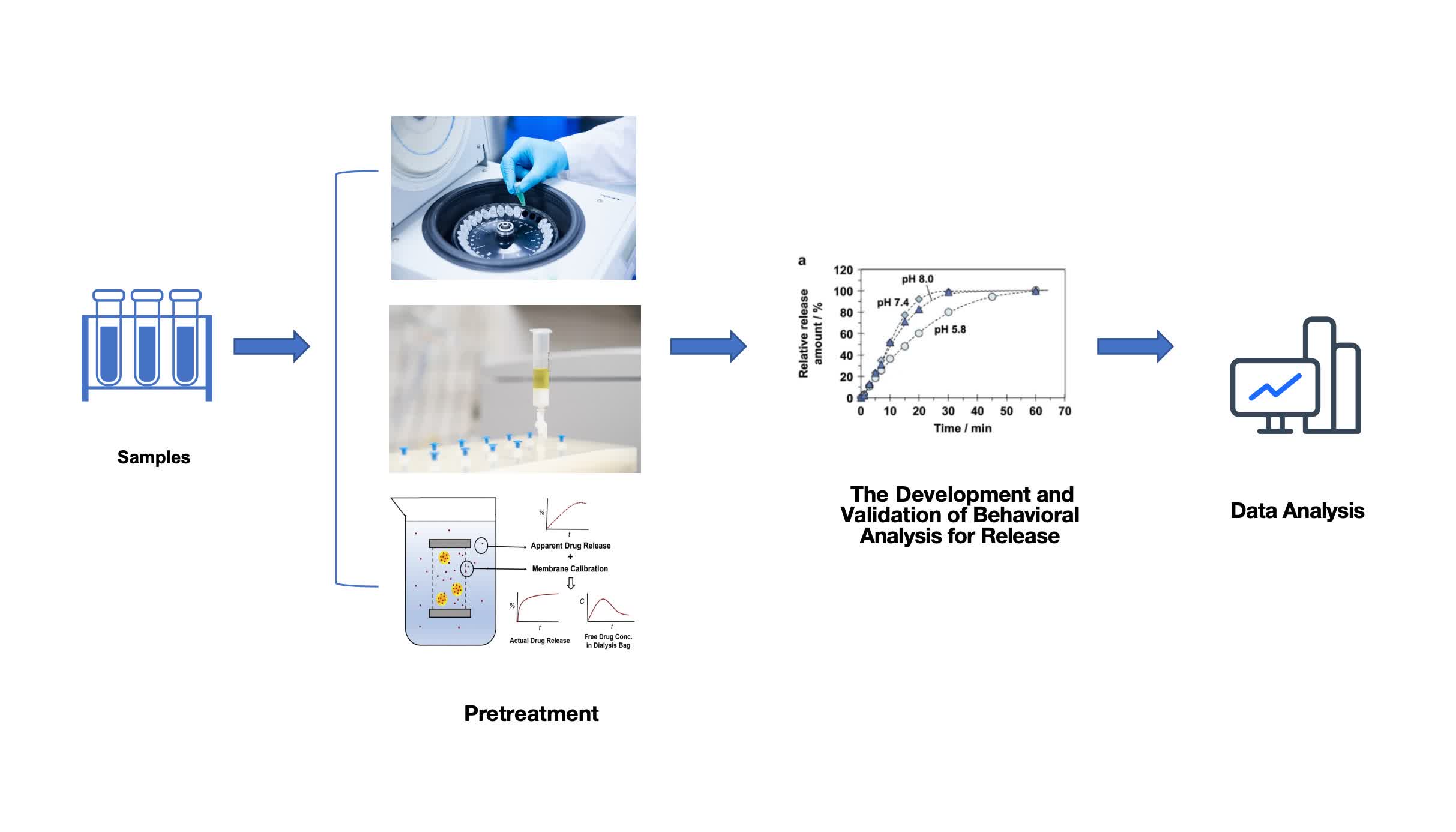 Fig. 1 The workflow of liposome in vitro release study. (CD Formulation)
Fig. 1 The workflow of liposome in vitro release study. (CD Formulation)
Our Platforms for Liposome In Vitro Release Study
| Techniques & Platform |
Advantages |
| Sample and separate methods |
- Device is more common, stirring method and sampling technology is simple.
- Relatively mature method.
|
| Forward dynamic dialysis method |
- Easy to operate and requires little equipment. No further filtration is required to separate liposomes and free drugs after sampling.
- The compatibility of drugs with dialysis bags and the adsorbability of dialysis bags affect the rate of liposome release.
|
| Reverse dynamic dialysis method |
- The use of dialysis bags has different sizes and shapes, and the interception of molecular weight, which affect the detection results.
|
| Ussing chamber method |
- Meets the needs of research on drug release behavior and tissue retention.
- Can be used to evaluate the release of liposomes in vitro, the membrane permeability of drugs and the retention of drugs in tissues.
- Non-injectable liposomes, such as liposome gels and topical liposomes, are widely used in the development of liposomes.
|
| Franz diffusing cells method |
- Low cost, convenient operation, no need to filter and separate after sampling, which can avoid drug loss.
- The stirring effect is difficult to ensure, and the bubbles are difficult to remove, which may lead to uneven distribution of the released drugs in the receiving room.
- Developed for in vitro study of drug release and transdermal effects of sterile semi-solid formulations, liposomes are not highly applicable to other dosage forms or other delivery routes.
|
| Flow through cell method |
- A reliable method for in vitro release analysis of complex liposome products, which is less affected by handling error.
- For the long-acting liposome preparation, the circulating flow cell method can effectively prevent the volatilization of the medium, meet the needs of the release determination in a long time, and effectively reduce the measurement error.
- The shortcomings of the equipment are high price, complex operation, the filter is easy to be blocked by foreign matter, and the filter and glass beads are easy to adsorb drugs.
|
Highlights for Liposome In Vitro Release Study
- Expertise. The members of our team consist of seasoned scientists and engineers who possess extensive expertise in vitro liposome release research.
- Tailored Solutions. We collaborate closely with our clients to gain a comprehensive understanding of their objectives about in vitro liposome release research and deliver optimal outcomes.
- Rigorous. The rigorous and verifiable quality standards about in vitro liposome release research is established for ensuring the provision of precise analysis results.
- Efficiency. The delivery of in vitro liposome release research test results within the specified time frame is ensured through effective project management and streamlined service processes.
Published Data
Technology: Green Synthesized Silver Nanoparticle-Loaded Liposome technology
Journal: Biomedicines
IF: 4.7
Published: 2023
Results: In this study, liposomes containing silver nanoparticles have been developed to effectively treat cancer. Silver nanoparticle encapsulated liposomes were prepared by thin film water method combined with ultrasonic treatment. The prepared liposomes were characterized by DLS (Dynamic Light scattering analysis), FESEM (Field emission scanning electron microscopy), and FTIR (Fourier transform infrared spectroscopy). In vitro drug release curves of silver nanoparticle liposomes were studied by dialysis bag method and verified by various mathematical models. The high encapsulation rate (82.25%) of silver nanoparticle supported liposomes was observed. In vitro drug release curves of silver nanoparticle supported liposomes were performed at three different pH values (pH 5.5, pH 6.8, and pH 7.4) using the dialysis bag method. High release of silver nanoparticles was observed at pH 5.5 from the Korsmeyer-Peppas model, confirming that drug release is based on abnormal non-fickian diffusion. The results show that liposomes loaded with silver nanoparticles can serve as effective drug delivery vehicles to target various types of cancer cells.
The in vitro release team at CD Formulation consists of a dedicated group of research scientists and data analysts with extensive experience collaborating with various companies and organizations to assess the release behavior of liposomes. If you need evaluation services of liposome release behavior in vitro, please feel free to contact us for further information.
References
- Jayachandran, P.; Ilango, S.; et al. Green Synthesized Silver Nanoparticle-Loaded Liposome-Based Nanoarchitectonics for Cancer Management: In Vitro Drug Release Analysis. Biomedicines. 2023, 11, 217.
How It Works
STEP 2
We'll email you to provide your quote and confirm order details if applicable.
STEP 3
Execute the project with real-time communication, and deliver the final report promptly.
Related Services



 Fig. 1 The workflow of liposome in vitro release study. (CD Formulation)
Fig. 1 The workflow of liposome in vitro release study. (CD Formulation)
