Liposome Biodistribution Study
Inquiry
Liposome biodistribution refers to the transport process between the blood and various tissues and organs after the liposome drugs enter the blood circulation, which has an important impact on the efficacy of the drug. CD Formulation is a global pharmaceutical company, and our liposome biodistribution study service focuses on the study of the distribution of liposomes in vivo. Through in vivo distribution studies of liposomal drugs, we help our clients assess the total drug exposure in various tissues, the concentration of free drugs in target tissues and other non-target tissues, and for locally-administered liposomal drugs, we focus on the distribution and accumulation of the local administration area and related organs or tissues.
Why You Need Liposome Biodistribution Study?
Drug distribution can cause adverse reactions and side effects. When liposome drugs are enriched in certain tissues or organs, it can lead to high local drug concentrations, which can produce toxic effects. The competitive binding of liposomal drugs with plasma proteins or histamins changes the distribution balance of drugs and affects drug efficacy or toxicity. Therefore, drug distribution is an important pharmacological process. The study on the biological distribution of liposome is very important for the initial stage of liposome drug development. The formulation of liposome drugs can be reverse-phase optimized according to the biological distribution characteristics in vivo, and the targeting of drugs can be improved significantly to improve the pharmacokinetic and metabolic characteristics of drugs in vivo, maximize the efficacy and reduce the incidence of side effects and toxicity.
Explore Our Services for Liposome Biodistribution Study
Our research center has established many years, and our services for liposome biodistribution are as following.
Study on factors affecting the biological distribution of liposomes
The type of drug distribution depends on physiological factors and physical and chemical properties of the drug, including tissue blood flow rate, physiological barrier, affinity between the drug and the tissue, fat solubility of the drug, and the binding of the drug to plasma proteins. We offer the following research services:
- Physical and chemical properties.
- Study on plasma protein binding rate.
- Apparent volume of distribution.
- absorption rate constant.
- Half-life study.
- Bioavailability study.
In vivo distribution PK test
Depending on the customer's requirements, the carrier and test items can be administered intravenously, intraperitoneally, orally or subcutaneously. At a specified time point (determined by the client) after administration, the animal is euthanized with CO2 and plasma and specific organ samples are collected. Plasma or organ samples are processed and analyzed by liquid chromatography-tandem mass spectrometry (LC-MS/MS) and a plasma or organ calibration curve for each compound is generated. To generate a plasma or organ calibration curve for each compound, a test compound with a specified concentration level is added to an equal sample of drug-free plasma or organ. Labeled plasma samples are processed using the same procedures as unknown plasma or organ samples to obtain a linear range and lower limit of quantitation (LLOQ) for the test.
Our Workflow of Liposome Biodistribution Study
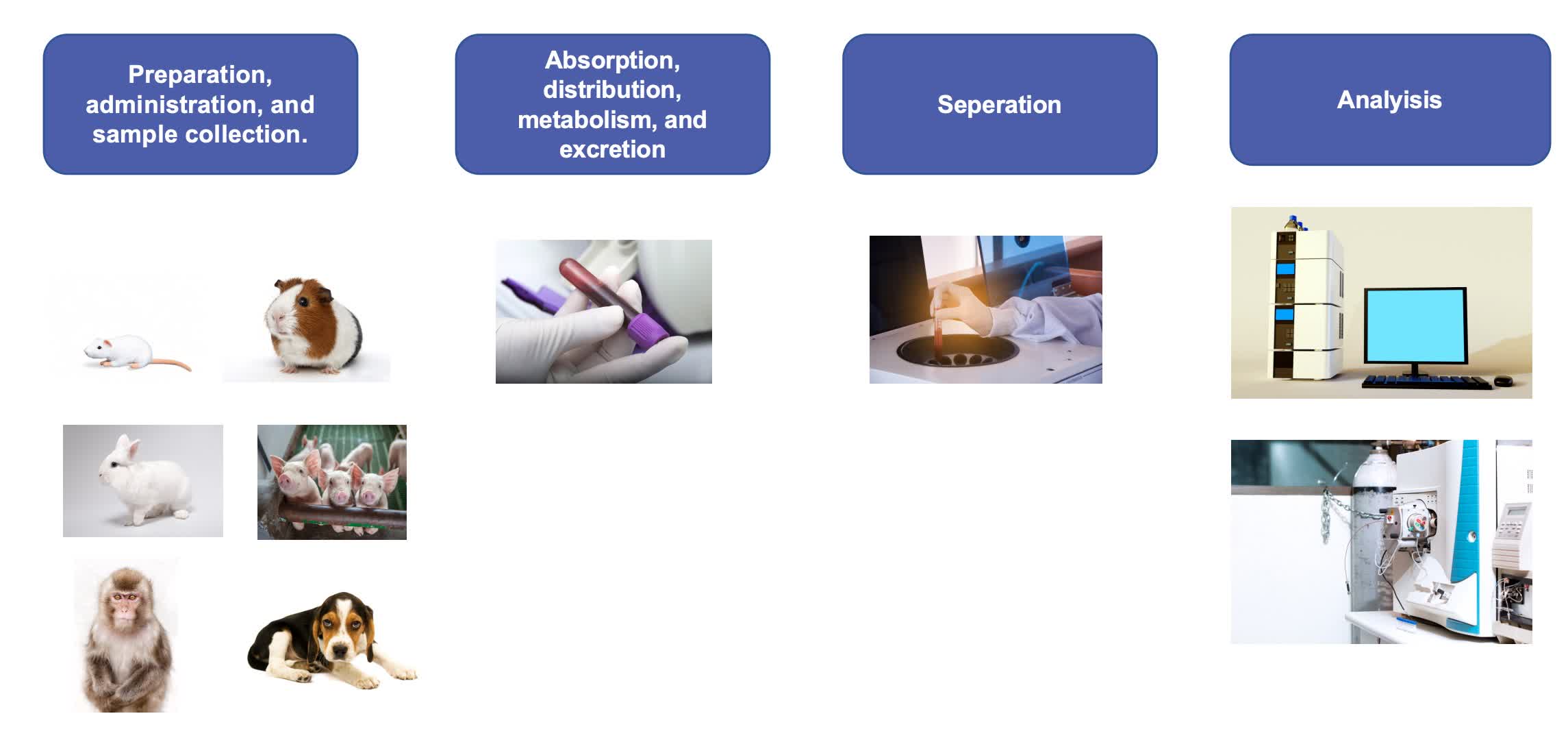 Fig.1 Our workflow for liposome biodistribution study. (CD Formulation)
Fig.1 Our workflow for liposome biodistribution study. (CD Formulation)
Our Platforms for Liposome Biodistribution Study
| Equipments & Platforms |
Specifics |
Pretreatment Platform

|
Verifiable pre-processing can be developed. We process following techniques:
- Solid phase extraction
- Ultrafiltration centrifugation
- Equilibrium dialysis
- Ultrafiltration centrifugation.
|
Drug Bioanalysis Platform

|
Our platform has dozens of liquid, mass spectrometry and high resolution analytical instruments, e.g.
- LC-MS/MS
- ELISA
- HPLC-UV
- Fluorescent labeling method
- Radiolabelling
- Magnetic resonance imaging.
|
Animal experiment platform

|
- 11,000 + mice, 2,500 + rats, and more than 200 rabbits, guinea pigs and large animals (dogs, pigs and monkeys) each.
- The personnel have laboratory animal professional technical certificate, veterinary practitioner qualification certificate, radiation safety and protection training certificate, etc.
|
Highlights for Liposome Biodistribution Study
- Strict quality management system. We possess ISO 17025 certification, Biosafety Level 2 (BSL-2) laboratory registration certificate, laboratory animal use license, radiation safety license, radioisotope registration certificate, etc. We have professional laboratory management system for liposomes biodistribution study.
- Empirical. Our laboratory staff have an average of many years of experience in conducting in vivo pharmacological research especially in liposome biodistribution study.
- Timely. Communicate fully with the customer before the project starts, respond quickly, and actively consider partners to advance the project.
Published Data
Technology: PEGylated liposomal technology
Journal: IET Nanobiotechnol
IF: 5
Published: 2022
Results: In this study, the biological distribution and antitumor activity of doxorubicin (PLD) preparations of pegylated liposomes of different sizes were investigated. First, PLDS with 100, 200, and 400 nm were prepared by remote loading procedures, and their sizes, zeta potentials, encapsulation efficiency, and release characteristics were characterized. In vitro cell uptake and cytotoxicity were then studied by flow cytometry and MTT assays and compared with commercially available PLD Caelyx ®. In vivo studies were applied to BALB/c mice with C26 colon cancer. Cytotoxicity and cell uptake tests did not show any statistically significant difference between PLDS. Biological distribution results showed that Caelyx ® and 100 nm liposome formulations accumulated the most doxorubicin (Dox) in tumor tissue and therefore significantly inhibited tumor growth compared to 200 and 400 nm PLD. In contrast, larger nanoparticles (200 and 400 nm preparations) accumulated more in the liver and spleen. This study shows that the 90 nm Caelyx ® biological distribution curve gives the drug stronger anti-tumor activity, resulting in significantly longer survival, and demonstrates the importance of vesicle size in nanoparticles targeting the tumor microenvironment to treat cancer.
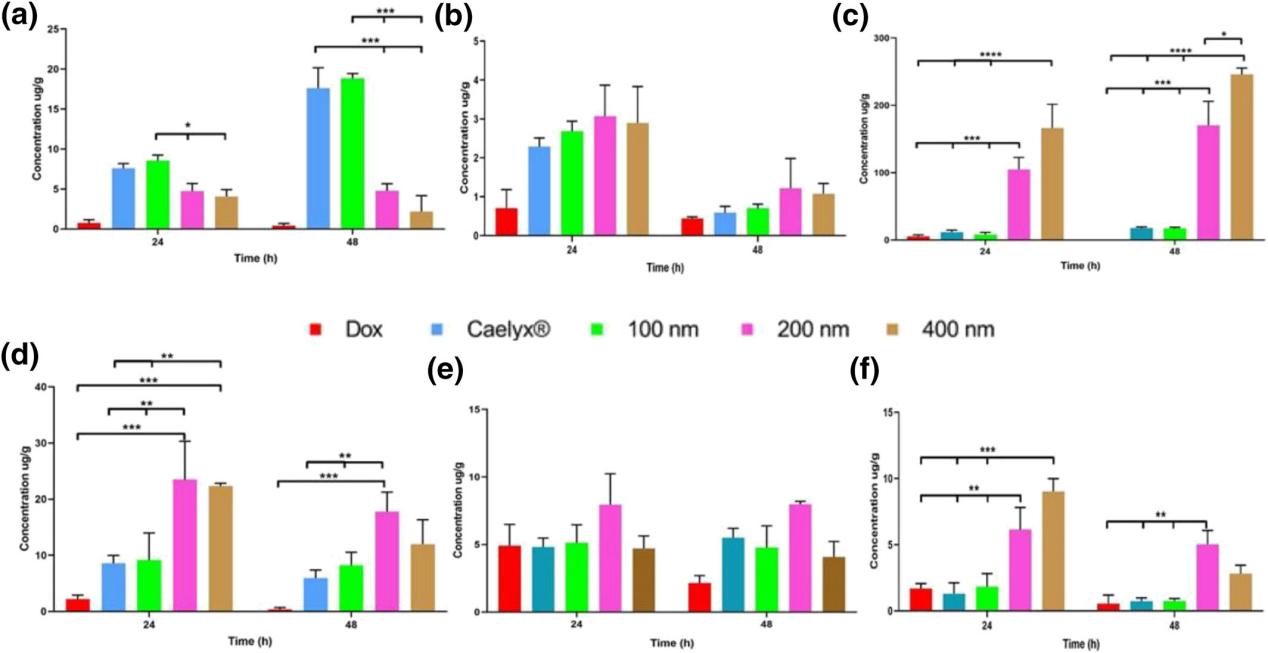 Fig.2 The process of apigenin-loaded stealth liposomes pharmacokinetic study. (Dadpour, S., et al., 2022)
Fig.2 The process of apigenin-loaded stealth liposomes pharmacokinetic study. (Dadpour, S., et al., 2022)
CD Formulation has accumulated many years of project experience in the study of the biological distribution of liposomes, and our team of scientists and experts are ready to answer your questions and provide advice. If you need our help, please contact us at your convenience.
References
- Dadpour, S., Amin Mehrabian, et al. The role of size in PEGylated liposomal doxorubicin biodistribution and anti-tumour activity. IET Nanobiotechnol. 2022, 16(7-8), 259–272.
How It Works
STEP 2
We'll email you to provide your quote and confirm order details if applicable.
STEP 3
Execute the project with real-time communication, and deliver the final report promptly.
Related Services


 Fig.1 Our workflow for liposome biodistribution study. (CD Formulation)
Fig.1 Our workflow for liposome biodistribution study. (CD Formulation)


 Fig.2 The process of apigenin-loaded stealth liposomes pharmacokinetic study. (Dadpour, S., et al., 2022)
Fig.2 The process of apigenin-loaded stealth liposomes pharmacokinetic study. (Dadpour, S., et al., 2022)
