Gene Therapy Formulation Process Development
Inquiry
Gene therapy formulation process development, designed to deliver gene therapy-related products with a high-quality level of quality. CD Formulation provides specialized process development services for gene therapy formulations. Our extensive experience ensures that we can provide reliable technical support for developing gene therapy formulations for our clients' projects. Further, accelerates the preclinical development and production of gene therapy products.
Importance of Gene Therapy Formulation Process Development
We provide gene therapy formulation process development that plays an important role in helping investigators accelerate the development and application of gene therapy drugs, including but not limited to the following.
- Effectively driving research progress in the industrialization of gene therapy formulation development.
- Provide a more cost-effective and robust way of manufacturing gene therapy formulations to improve product safety and reliability.
- Optimize the process flow and reduce possible operational errors during development and manufacturing.
- Help customers to greatly shorten the time from development to clinical trials and commercialization of their gene therapy formulations.
Explore Our Gene Therapy Formulation Process Development
Our services include, but are not limited to cell culture process development, viral vector packaging, viral vector purification, viral vector characterization, and so on. Specific services are shown below.
Cell Culture Process Development
We believe that our cell culture process development services, including adherence culture, suspension culture, microcarrier culture, etc., can help our customers select more appropriate cell culture processes to facilitate large-scale viral vector production or other beneficial aspects.
Viral Vectors Packaging
We continuously optimize our technology system, aiming to provide the most advanced viral vector packaging service. Our viral vector packaging services include but are not limited to, adenovirus packaging, adeno-associated virus (AAV), lentivirus, and so on.
Viral Vector Purification
In the development of gene therapy formulations, different viruses have different characteristics and therefore require different purification methods. We provide viral vector purification solutions that can maximize our customers' vector purification research needs and improve the infectivity of viruses.
Viral Vector Characterization
Our advanced technology platform supports viral vector characterization, and we provide researchers with comprehensive viral vector characterization services, including viral titer, viral purity, viral particle size, and distribution.
Gene Therapy Starting Raw Material Preparation
It is important to note that we also provide raw material preparation services for gene therapy formulation process development as well as a range of services such as raw material risk assessment and quality control. These services ensure that no viruses or other contaminants are introduced into the raw materials during the manufacturing process.
Gene Therapy Manufacturing Process Development
We provide a full range of gene therapy formulation process development services, such as target gene identification, vector construction, gene synthesis and purification, and a series of other services. This service plays an important role in helping researchers improve the safety and efficacy of gene therapy formulations.
Our Process of Gene Therapy Formulation Process Development
- Target gene selection and design. After determining the target disease for gene therapy, appropriate genes are selected for design and optimization to ensure their effective expression in the target cells.
- Vector selection. According to the characteristics of the project, select suitable gene vectors, such as viral vectors or non-viral vectors. In vector selection, various factors need to be considered, such as transduction efficiency, safety and durability of the vector.
- Cell culture and expansion. Selection of suitable cell lines for gene transfection or transduction also requires cell culture to generate sufficient vector or transfected cells.
- Gene transduction. The designed genes are transduced into the target cells by the selected vectors, and subsequent observation of the
- transduction efficiency and changes in gene expression levels.
- Formulation development. This segment requires the optimization of gene therapy formulation, including including stability, solubility, etc., and also the selection of appropriate delivery modes, such as injection and topical administration.
- Quality control. In order to further ensure the purity, safety and biological activity of gene therapy formulations, a series of quality tests are also required.
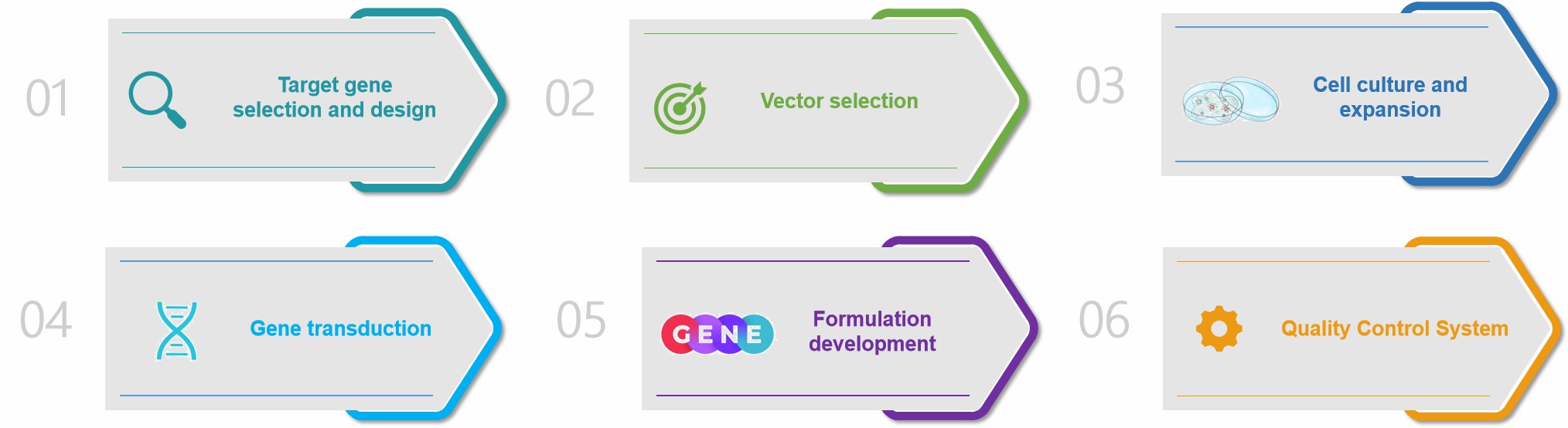 Fig.2 Our process of gene therapy formulation process development . (CD Formulation)
Fig.2 Our process of gene therapy formulation process development . (CD Formulation)
Our Platforms for Gene Therapy Formulation Process Development
| Technologies & Platforms |
Content Description |
| Viral vector production platform |
Our advanced viral vector production platforms, including adeno-associated viruses (AAVs), lentiviruses (LVs) and retroviruses (RVs), play an important role in improving the yield and quality of viral vectors. |
| Cell culture platforms |
This technology platform of ours is capable of dramatically increasing cell growth and viral vector production through the use of advanced bioreactors and culture media. It is as if our suspension cell culture platform can be used for large-scale viral vector production, effectively increasing productivity and reducing costs. |
| Purification and formulation platforms |
We use advanced purification technologies, such as chromatography and ultrafiltration, to remove impurities and contaminants and improve the purity and activity of our viral vectors. This technology platform further ensures the stability and storability of the gene therapy formulations we develop. In addition, our gene therapy formulation platform utilizes the latest formulation technology and equipment to develop specific gene therapy formulations based on the characteristics and requirements of our client's projects. |
Highlights of Our Gene Therapy Formulation Process Development
- Our expert technical team has many years of experience in gene therapy formulation development and can optimize the entire development process.
- Our technology platform and service standards comply with the regulatory requirements of the relevant organizations, and working with us ensures high-quality services are offered.
- We can provide customized gene therapy formulation process development services according to the characteristics and requirements of our clients' projects.
- We have a strict quality control system to ensure high-quality and high-standard process development services are offered.
With years of experience in gene therapy formulation process development and a strong team of experts and technicians, CD Formulation provides high therapeutic and high-standard gene therapy formulation process development. If you are interested in us, please feel free to contact us.
Related Services


 Fig.2 Our process of gene therapy formulation process development . (CD Formulation)
Fig.2 Our process of gene therapy formulation process development . (CD Formulation) 