Cell Culture Process Development
Inquiry
Gene therapy involves the insertion, removal or alteration of therapeutic or working gene duplicates to cure a disease or defect or slow down the progression of a disease, thereby improving the quality of life. Gene therapy holds great promise for addressing human health and disease, with the development of cell culture technology being particularly important in advancing gene therapy research. CD Formulation is an industry leader in gene therapy formulation development. Based on our state-of-the-art technology platform and experienced team of experts, we provide comprehensive technical services for cell culture process development, including optimization of the culture process to ensure access to high titer, high yield viral production, etc., in support of gene therapy research.
Importance of Cell Culture Process Development
Improvement of therapeutic efficacy. Optimizing cell culture conditions can effectively improve cell activity and function, which plays an important role in improving the therapeutic effect of gene therapy.
- Higher safety. The development of cell culture processes requires stringent quality control to further ensure the safety, purity and stability of cell products.
- Higher productivity. Cell culture process optimization and development increases productivity, reduces human error, and ensures a consistent and reproducible production process.
- Meet a wide range of market needs. The development of cell culture processes facilitates the transition from lab-scale to industrial production, meeting a wider range of market needs.
- More economically viable. By optimizing cell culture processes, production costs can be reduced, making gene therapy more economically viable.

Explore Our Cell Culture
In gene therapy programs, viruses such as AAV are produced by transfecting the appropriate host cells. The current production methods are mainly adherent culture, microcarrier culture, and suspension culture. Due to the fundamental differences in culture methods between adherent cells and suspension cells, such as cell morphology, metabolism, growth behavior, etc., the quality and yield of the target product will be greatly affected. Therefore, the selection of an appropriate cell culture process is of increasing importance for large-scale viral vector production.
Adherent wall culture
Adherent wall culture. Taking cell factories as an example, cell factories can save more manpower, material resources, time, and space compared with traditional cell flasks, with a low risk of contamination, and can realize large-scale cell culture. For example, in poxvirus research, we have improved the virus titer by 10-fold and the single-cell yield up to 260 PFU/cell through the continuous optimization of the process, which improves the yield and reduces the production cost at the same time, and verifies that the optimized virus production process is suitable for large-scale production.
Suspension culture
Suspension culture is easy to realize the whole process of closed operation, the process of aseptic control is simple, easy to take samples, culture operation is simple and controllable, easy to amplify, contamination rate and low cost. Serum-free culture can be realized, reducing the risk of microbial contamination such as viruses, fungi, and mycoplasma brought by serum. Taking recombinant adenovirus as an example, we use serum-free suspension cell culture, and after optimizing several key factors, we can significantly increase the viral titer and yield, and effectively realize large-scale serum-free suspension culture of adenovirus vectors.
Microcarrier culture
Microcarrier culture combines the advantages of both adherent and suspension cultures, providing a large culture surface area/volume ratio, high cell yields without the need for high-capacity equipment, and allowing cells to be cultured in suspension in the adherent state. For wall-dependent cell cultures, microcarrier cultures require considerably less space to produce a given amount of cells or cell products, reducing both labor requirements and the risk of contamination.
General Workflow of Our Cell Culture Process Development
Cell separation and enrichment
We isolate and enrich cells according to the experimental goal. For example, by leukocyte isolation, we can collect peripheral blood mononuclear cells from patients, and isolate and enrich target cells, such as hematopoietic stem cells and T cells.
Cell expansion
The collected cells need to be expanded to obtain a sufficient number of cells for subsequent processing and preparation.
Cell transfection
Transfection of constructed gene vectors into host cells, mainly using viral vectors, but also chemical and electroporation transfection.
Cell culture
The transfected cells are cultured and expanded to obtain a sufficient number of cells. In the process of cell culture, we can choose different culture methods according to different types of viruses, experimental characteristics and objectives, etc.
Cell isolation and purification
Isolation and purification of cells in gene therapy removes other cell types, impurities and components from the culture.
Quality control and quality assurance
Quality control and quality assurance of the prepared cellular gene therapy drug to ensure that it meets the standards of safety and efficacy.
Applications of Our Cell Culture Process Development
- Cancer therapy. By culturing modified immune cells, such as CAR-T cells, for recognizing and attacking cancer cells.
- Genetic disease treatment. Using cell culture technology, normal genes are introduced into the patient's cells to correct or compensate for genetic defects to treat diseases such as cystic fibrosis and sickle cell anemia.
- Cardiovascular disease treatment. Culturing cardiomyocytes or vascular endothelial cells for repairing damaged heart tissue or blood vessels.
- Neurodegenerative disease treatment. Cultivation of neural stem cells or specific types of neural cells for the treatment of Parkinson's disease, Alzheimer's disease, etc.
- Diabetes treatment. Cultivation of pancreatic beta cells or insulin-producing cells for replacing damaged pancreatic islet cells to treat type 1 diabetes.
Our Platforms for Cell Culture Process Development
| Platforms & Technologies |
Content Description |
| Gene editing technologies |
We have established gene editing technology platforms including zinc finger nuclease (ZFN) technology, transcription activator-like effector nuclease (TALEN) technology, regularly interspaced clusters of short palindromic repeats (CRISPR) technology, and base editing technology to enable precise modification of cellular genomes, correction of genetic defects, or enhancement of cellular functions. |
| CAR-T cell technology |
This is an immune cell therapy technology in which T cells are genetically engineered to become chimeric antigen receptor T cells (CAR-T) so that they can specifically recognize and kill tumor cells. |
| 3D cell culture technology |
This technology allows cells to grow in three dimensions, mimicking the in vivo environment, which is important for studying cell behavior and drug screening. |
Highlights of Our Cell Culture Process Development
- We have an advanced cell culture technology platform that ensures optimized cell culture solutions.
- We can provide customized cell culture process development services based on customer requirements and research objectives.
- We provide full-process services for cell culture in gene therapy, effectively helping researchers to solve the challenges in cell culture.
- We implement strict quality control to ensure the accuracy of our technical services.
Published Data
Technology: Development of a suspension HEK293 cell line
Journal: Mol Ther
IF: 4.7
Published: 2015
In this study, the researchers worked on scalable manufacturing processes to efficiently generate high titers, high purity, and effective amounts of recombinant adeno-associated virus (rAAV) vectors. Utilizing a relatively simple and efficient HEK293 cell transfection system as a starting point, an adherent HEK293 cell line derived from a qualified clinical master cell bank was successfully adapted for rapid and scalable rAAV production by growing it in shake flasks and WAVE bioreactors under suspension conditions without animal components.
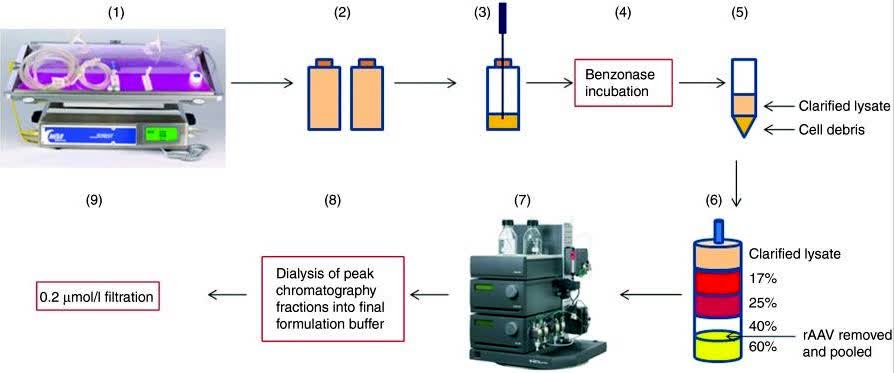 Fig.2 Depiction of recombinant adeno-associated virus (rAAV) manufacturing technology. (Grieger JC, et al., 2015)
Fig.2 Depiction of recombinant adeno-associated virus (rAAV) manufacturing technology. (Grieger JC, et al., 2015)
CD Formulation is an industry leader in the development of gene therapy formulations, and we offer comprehensive technical services for cell culture process development. If you are interested in us, please feel free to contact us.
References
- Grieger JC, et al. Production of Recombinant Adeno-associated Virus Vectors Using Suspension HEK293 Cells and Continuous Harvest of Vector From the Culture Media for GMP FIX and FLT1 Clinical Vector. Mol Ther. 2016, 24(2):287-297.
Related Services


 Fig.2 Depiction of recombinant adeno-associated virus (rAAV) manufacturing technology. (Grieger JC, et al., 2015)
Fig.2 Depiction of recombinant adeno-associated virus (rAAV) manufacturing technology. (Grieger JC, et al., 2015)