Gene therapy involves the transfer of genetic material into the patient's cells to achieve a therapeutic effect. Viral vector systems used for therapeutic gene delivery include adenoviruses, adeno-associated viruses, and lentiviruses. As the use of clinical therapeutics such as viral vectors grows, it is important to investigate and optimize processes that can be used for scale-up to better preserve the critical quality attributes of the viral vectors. CD Formulation is dedicated to providing purification process methodologies and total solutions based on a wide range of gene therapy viral vectors to address the challenges you face in the development of your gene therapy formulations.
Features of Viral Vector Purification
- Multi-step process. Purifying viral vectors usually involves a multi-step process including cell filtrate collection, removal of cellular debris and cellular proteins, enrichment, further purification, and viral vector concentration.
- Technological diversity. Various chromatographic techniques, such as ion exchange chromatography, affinity chromatography, gel filtration chromatography, etc., are used to improve the purity and concentration of viral vectors.
- Mild conditions. Chromatography is one of the main techniques due to its mild process, high resolution, good selectivity, and easy amplification.
Explore Our Viral Vector Purification
Synthesis and Optimization of Polymeric Carriers
The downstream purification process for the viral vectors we offer includes cell filtrate collection, removal of cellular debris and cellular proteins, viral vector enrichment, further purification, and viral vector concentration. These steps are critical to ensure the quality and efficacy of the final gene therapy product.
Cell filtrate collection
Cell filtrate collection is the first step in the viral vector purification process and is critical because it involves the collection of viral particles from host cell cultures containing viral vectors.
Removal of cellular debris and cellular proteins
The lysed mixture needs to be centrifuged or filtered to remove cellular debris and unlysed cells.
Viral vector enrichment
Viral vectors are usually enriched using ultrafiltration or precipitation techniques.
Initial purification and further purification
Preliminary separation by chromatographic techniques utilizing the properties of the viral vectors. Commonly used chromatographic methods include ion exchange chromatography, hydrophobic interaction chromatography, and affinity chromatography. The purity of the viral vector is further improved using size exclusion chromatography (SEC) or other specific chromatographic techniques. Subsequently, further vector purification can be performed, to increase the purity of the viral vectors and to remove the remaining host cell proteins, DNA and other impurities.
Viral vectors concentration
Concentration of viral vectors is a key step in the purification process to increase the concentration of viral vectors for subsequent purification, storage and application.
Our Solutions for Viral Vector Purification
Different viruses in gene therapy have different properties and therefore the corresponding purification methods need to be adapted accordingly. We optimize viral vectors to maximize viral infectivity.
AAV can also be purified using IEX, as well as heparin affinity chromatography, which is particularly suited for AAV2. SEC is suitable for further purification of AAV5. The AAV purification process needs to be optimized to maintain viral infectivity and maximize recovery.
Adenovirus purification can be performed using a variety of chromatographic methods including anion exchange chromatography (IEX), affinity chromatography (AF), size exclusion chromatography (SEC), and hydrophobic interaction chromatography (HIC). Because adenoviruses are negatively charged at neutral pH, they can be purified using anion-exchange adsorbents. A scalable adenovirus production process from upstream cell culture to downstream purification using modern tools and techniques is easy to scale up and is compatible with disposable hard pipeline process equipment that can be vaporized.
Lentiviruses are routinely purified using membrane adsorbers and column-based cation exchange (AEX) tools used in the capture step. In addition, lentiviruses can also be purified by using heparin affinity chromatography. Besides, lentiviral vectors can be purified by size exclusion chromatography (SEC), a method that efficiently separates viral vectors from other proteins and impurities.
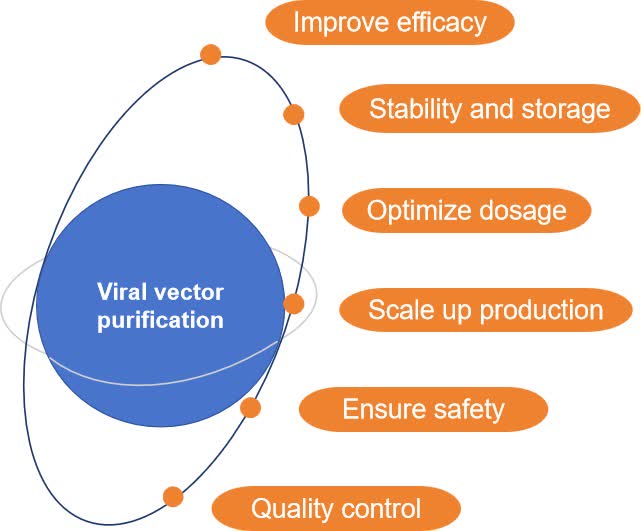 Fig.1 Role of viral vector purification. (CD Formulation)
Fig.1 Role of viral vector purification. (CD Formulation)
- Reduce immune response. Removal of impurity proteins and other potentially immunogenic substances can reduce the patient's immune response and improve the safety of the therapy.
- Ensure safety. Viral vector purification helps to remove potentially harmful substances, thereby ensuring the safety of the final product.
- Optimize dosage. High-purity viral vectors allow for more accurate determination of therapeutic doses and avoid adverse reactions caused by impurities.
- Stability and storage. Purified viral vectors are easier to store and transport because of their more homogeneous composition and better stability.
- Scale-up production. Scale-up production of viral vectors relies on efficient and reproducible purification processes.
Our Platforms for Viral Vector Purification
| Platforms & Technologies |
Content Description |
| Chromatographic methods |
Classical large-scale purification techniques are highly suitable for developing gene therapy products. Chromatographic methods can be further categorized into four modes, each utilizing a different mechanism for virus purification. |
| Ultracentrifugation purification techniques |
Primarily used on a laboratory scale and are not scalable because the available rotors are usually of small capacity. |
| Membrane adsorber |
Membrane adsorbents with membrane pore sizes >3 μm have become more commonly used for virus purification. They adsorb virus particles into the solid phase. Due to their large pore size, they can be run at faster flow rates, resulting in significant time savings and cost reductions (CoG). Another advantage is that they can be used with mild elution conditions, which better ensures virus infectivity. |
Highlights of Our Viral Vector Purification
- We can provide targeted vector purification technology services based on the characteristics of the target viral vectors.
- We carry out strict quality control throughout the vector purification process to ensure the reliability of the final product.
- We have perfect pre-sales and after-sales service, which can satisfy customers' vector purification research needs to the greatest extent.
- We can provide customized viral vector purification solutions according to customer requirements.
Published Data
Technology: AAV purification
Journal: Biotechnol Bioeng
IF: 3.8
Published: 2020
In recent years, there has been a strong interest in the development and production of gene therapy products, particularly those utilizing adeno-associated virus (AAV) particles, as evidenced by the growing number of clinical successes and institutional approvals for AAV therapies. Due to increased investment in the technology, scalable commercial manufacturing methods are needed to ensure adequate product availability as AAV research moves from laboratory development to clinical production. This study summarizes the scalable purification technologies currently available for commercial manufacturing of AAV and highlights certain development considerations, such as the use of quality-by-design principles for exclusion factors and process development.
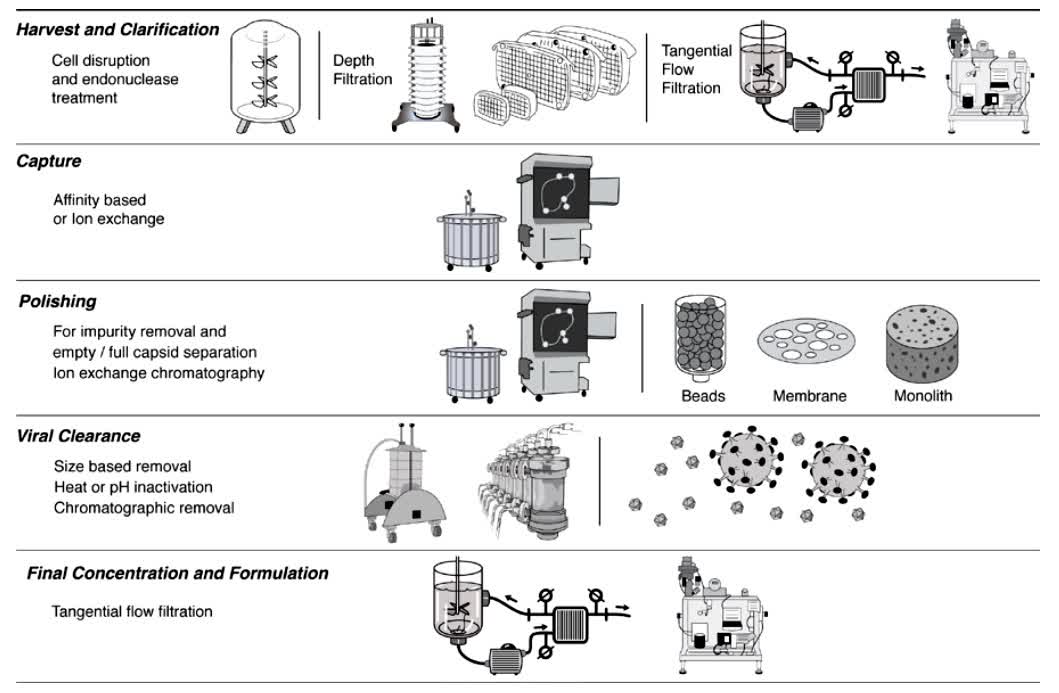 Fig.2 General schematic of a typical downstream adeno-associated virus purification process. (Adams B, et al., 2020)
Fig.2 General schematic of a typical downstream adeno-associated virus purification process. (Adams B, et al., 2020)
CD Formulation provides reliable technical support and solutions for viral vector purification, and this service plays an important role in the advancement of gene therapy. If you are interested in us, please feel free to contact us.
References
- Adams B, et al. Moving from the bench towards a large scale, industrial platform process for adeno-associated viral vector purification. Biotechnol Bioeng. 2020, 117(10):3199-3211.
Related Services

 Fig.1 Role of viral vector purification. (CD Formulation)
Fig.1 Role of viral vector purification. (CD Formulation) Fig.2 General schematic of a typical downstream adeno-associated virus purification process. (Adams B, et al., 2020)
Fig.2 General schematic of a typical downstream adeno-associated virus purification process. (Adams B, et al., 2020)