Viral vectors are replication-defective vectors obtained after modification, which can carry exogenous genes and packaged into viral particles, and the gene genetic material carried by the virus enters the cells for infection, and the viruses have a high infection efficiency, and can infect both dividing and non-dividing cells. Viral vectors, as a tool for gene delivery, have shown good application in the field of gene therapy.
CD Formulation is an industry leader in viral packaging services. We always focus on the needs of our customers and provide them with one-stop services from gene synthesis to viral packaging in order to assist their gene therapy formulation development projects.
Technical Advantages of Viral Vector Packaging
- Mature and industrializable. Virus packaging technology has matured after years of research and can be used for industrialized mass production.
- Simple genome structure and easy to modify. Viral genome structure is simple, clear molecular background, stable and easy to modify and prepare.
- Wide host range and target specificity. Viral vectors have a wide range of hosts and high target specificity.
- High safety. Engineered viruses are generally replication-defective, and high safety is achieved by forming a replication-defective structure by means of vector modification.
Explore Our Viral Vector Packaging
Our services focus on the use of genetic engineering to modify viruses and transform them into vectors capable of carrying exogenous genes. These modified viruses can infect cells and carry genes into them for long-term expression. Our viral vector packaging services play a critical role in the development of gene therapy formulations, which not only improve the efficiency and safety of gene delivery but also provide the possibility of long-term stable gene expression. To help our customers accelerate the development process of gene therapy formulations, we have continuously optimized our technology system, aiming to provide cutting-edge viral vector packaging services. In this process, we always put the safety and efficacy of our formulations in the first place. We can also customize the production of various viral vectors, including adeno-associated virus (AAV) and lentiviral vectors, according to the needs of our customers.
Our dedicated team will assist you in your viral vector development efforts, providing customized AAV and lentiviral packaging services for different study phases and scales. We provide comprehensive testing services to ensure high-quality viral vectors, including but not limited to testing for empty shell rate, endotoxin levels, sterility, and mycoplasma, to ensure you get stable, pure, and high-yield viral vectors to optimize performance for superior therapeutic outcomes.
We customize viral vector packaging services
Our Process for Viral Vector Packaging
- Viral vector construction. The process of creating a viral expression plasmid with the target gene, which often includes the packaging signals and viral sequences required to guarantee that the viral RNA can be effectively packaged into viral particles.
- Production of packaging plasmids. Preparation of packaging plasmids carrying genes encoding viral structural proteins that are responsible for the formation of the capsid and necessary enzymes for viral particles.
- Packaging cell culture and transfection. The constructed viral vectors and packaging plasmids are transfected into packaging cells, and using chemical or physical transfection methods, the expression and packaging plasmids are co-transfected into specific cells, which read the information from these plasmids and begin production of virus particles.
- Collection of viral particles. Sometime after transfection, the cells release viral particles containing the target gene into the culture medium, which is obtained by collecting the culture supernatant of the host cells.
- Virus concentration and purification. The obtained viral supernatant usually contains a large amount of cellular debris and protein impurities, which require specific purification methods to concentrate and purify the viral particles.
- Virus titer assay. After virus concentration and purification, viral titers need to be determined to ensure that valid and consistent viral concentrations are used in subsequent experiments.
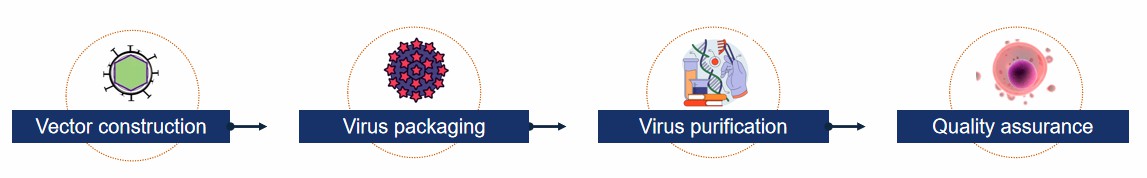 Fig.2 Viral vector packaging process. (CD Formulation)
Fig.2 Viral vector packaging process. (CD Formulation)
Our Platforms for Viral Vector Packaging
| Technologies & Platforms |
Content Description |
| Directed evolution technology |
Directed evolution technology stands as a remarkable tool, enabling the optimization of viral vector performance by mimicking the rigorous process of natural selection. It works by identifying viral particles that exhibit heightened efficiency in production and delivery. Through this innovative approach, engineered virus-like particles have undergone profound improvements, yielding vectors with vastly enhanced production rates and more effective delivery mechanisms. |
| Lentiviral vector construction technology |
Lentiviral vectors are particularly prized for their ability to integrate seamlessly into the host cell genome, facilitating the long-term expression of therapeutic genes. However, their construction is a delicate dance of precision—ensuring that the vectors are not only safe and non-toxic but also highly efficient and specific in their gene delivery. This process demands meticulous molecular cloning, the insertion of therapeutic genes, and, perhaps most critically, the removal or attenuation of any viral sequences that could present a risk of pathogenicity. |
| Viral packaging technology |
Viral packaging technology plays a pivotal role in gene delivery research. It is through the careful insertion of target genes into viral vectors that essential breakthroughs in gene therapy, gene editing, and other forms of genetic manipulation are achieved. Begins with the construction of gene vectors, followed by the transfection of cells, the extraction of viral particles, and their subsequent purification. At every step, the functionality of these viral particles must be verified to ensure their efficacy in research applications. |
Highlights of Our Viral Vector Packaging
- High-quality viral vectors. We provide high-quality and high-purity viruses that meet the standards for cellular infection to ensure the reliability and validity of experiments.
- Efficient and on time. We guarantee that our projects are completed on schedule to satisfy our clients' expectations by using a proven project management procedure and stringent quality control.
- One-stop service. We provide a full range of services from gene synthesis to virus packaging to simplify the research process for our customers.
- Diversified vector selection. Customers can choose from a wide range of vectors and labels to meet the specific needs of different experiments.
Published Data
Technology: Lentiviral packaging
Journal: J Gene Med
IF: 3.1
Published: 2004
Retroviral-based gene transfer vectors provide an effective means for the delivery, integration and expression of exogenous genes in mammalian cells. Lentiviral (LV) vectors provide attractive gene delivery vectors in a non-dividing cell environment. This article describes the different optimized LV genetic systems to date, including the production of LV-derived vectors all including genetically dividing gene expression designs, and further discusses the genetic requirements and performance of lentiviral viral elements.
CD Formulation offers viral vector packaging services that are engineered and optimized to greatly enhance biosafety. If you are interested in us, please feel free to contact us.
References
-
Delenda C. Lentiviral vectors: optimization of packaging, transduction and gene expression. J Gene Med. 2004, 6 Suppl 1:S125-38.
Related Services


 Fig.2 Viral vector packaging process. (CD Formulation)
Fig.2 Viral vector packaging process. (CD Formulation)