Gene Therapy Manufacturing Process Development
Inquiry
Gene therapy, as an innovative medical treatment to cure diseases by modifying or replacing abnormal genes in patients, has been progressing rapidly in the biomedical field this year. The validation of the multi-gene therapy manufacturing process plays an important role in guaranteeing the safety and efficacy of the product. CD Formulation, as your trusted life science expert, provides comprehensive technical support and effective solutions for the development of manufacturing processes in gene therapy. Our extensive experience enables us to develop robust manufacturing processes. Partner with us and let us help you accelerate your gene therapy product research.
The Importance of Gene Therapy Manufacturing Process Development
- Ensure product quality. Manufacturing processes for gene therapy products are developed to ensure that gene therapy products meet stringent regulatory requirements, including safety, authentication, purity, specifications and potency.
- Meet research needs. With the rapid development and increased clinical use of gene therapy products, there is a growing need for large-scale production, which can be met by optimizing the manufacturing process to meet research needs.
- Improve process efficiency. By optimizing steps such as cell culture methods, lysis processes, tangential flow filtration (TFF), and chromatography processes, it is possible to increase the yield and purity of gene therapy products while reducing production costs.
- Transition from laboratory to clinical production. Gene therapy products are initially developed at the laboratory scale, and further development and optimization of the manufacturing process is required to meet the requirements of clinical trials of gene therapy products.

Our Services for Gene Therapy Manufacturing Process Development
Gene acquisition and modification
The first step in gene therapy is to acquire the target gene. This usually involves in vitro cloning techniques of the DNA, including steps such as PCR amplification, enzymatic cleavage, and ligation. Modification of the gene, such as precision editing using the CRISPR/Cas9 system, may then be required to achieve a specific function or to eliminate harmful effects.
Vector construction
Genes need to be delivered into a cell to be therapeutic, which requires a vector. Common vectors are viral vectors (e.g. adenovirus) and non-viral vectors (e.g. lipid nanoparticles). The selection and construction of vectors is an important part of the gene therapy process, which needs to ensure the safety, stability and efficient transduction ability of the vector.
Gene production and purification
After the modified gene is inserted into the vector, large-scale gene production begins. This is usually carried out in a bioreactor, where the gene is expressed by host cells (e.g. E. coli or mammalian cells). Impurities are then removed through a series of isolation and purification steps, such as centrifugation, ultrafiltration, and chromatography, to obtain a highly pure gene product.
Quality control
Strict quality control is essential in the production of gene products. This includes validation of gene sequences, vector stability testing, sterility checks, and activity assays to ensure product quality and safety.
Preparation and packaging
Finally, gene products are prepared into forms suitable for clinical application, such as lyophilized powders or solutions, and aseptically encapsulated to ensure their stability during storage and transportation.
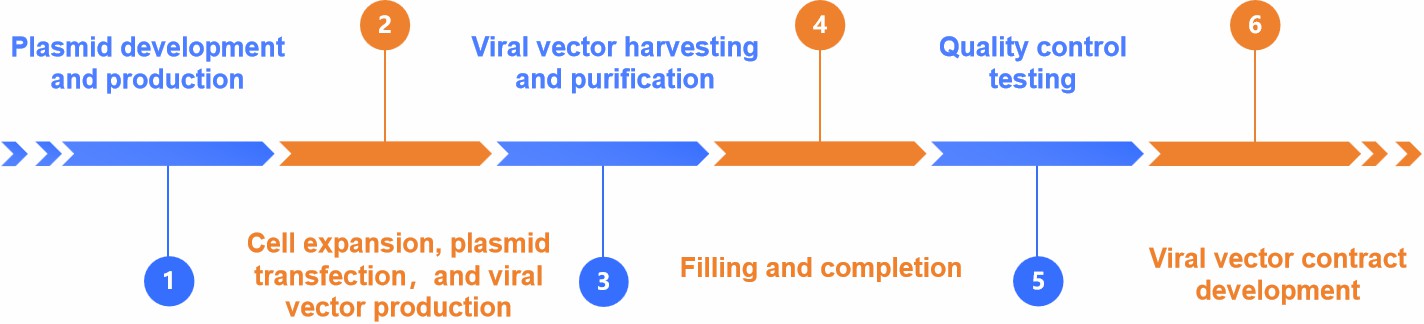 Fig.2 Gene therapy manufacturing process. (CD Formulation)
Fig.2 Gene therapy manufacturing process. (CD Formulation)
Gene therapy products are made through an intricate and complicated process, where every stage has a direct impact on the final product's effectiveness and quality. Our goal is to contribute to further innovations and breakthroughs in the field of gene therapy and greater benefits for human health through ongoing process optimization and technical service improvement.
Our Platforms for Gene Therapy Manufacturing Process Development
| Platforms & Technologies |
Content Description |
| Viral vector optimization technology |
Viral vectors, including adeno-associated virus vectors, are key tools in gene therapy. We have established a viral vector optimization technology platform to continuously improve the safety, efficacy and targeting of these vectors. |
| CRISPR-Cas9 gene editing technology |
This technology enables the knockout of genes or the introduction of small sequence changes by cutting the DNA double-strand using a nuclease such as Cas9. CRISPR/Cas9 and related gene editing technologies play an important role in the field of gene therapy research. |
| Chromatography-free plasmid process |
We provide a process technology that not only accomplishes a high-quality plasmid result by lowering the complexity of the process in terms of equipment, consumables, and other related factors but also streamlines the process by comprehending the critical process parameters. |
Highlights of Our Gene Therapy Manufacturing Process Development
- We have the technical expertise to shorten your project's research time and help you improve your research efficiency.
- We provide customized solutions to meet your needs in gene therapy process development according to your project requirements.
- We are constantly updating our knowledge and technology system, aiming to provide you with the most professional and advanced technical services.
- Our gene therapy manufacturing process development services cover a wide range of areas from vector design to quality control of gene therapy products.
Published Data
Technology: Production of synthetic AAV gene therapy vectors
Journal: Biotechnol J
IF: 5.7
Published: 2021
In the past 20 years, AAV-based gene therapy vectors have proven to be safe and effective, leading to the approval of three gene therapy products. Preclinical studies have also highlighted the advantages of synthetic AAV capsids, which offer improved specificity and efficiency in gene transfer, as well as reduced immune responses. This has driven the need for scalable and adaptable processes that can handle the growing variety of AAV capsid variants. Recent developments in downstream processing and characterization methods, including various chromatography techniques and thermostability testing, are being explored. The article uses AAV-DJ and Anc80 as examples of chimeric capsids evolved through molecular techniques. These advancements are expected to streamline the development and manufacturing of the next generation of AAV vectors, thereby advancing the field of gene therapy.
CD Formulation provides a full range of gene therapy process development services to improve the safety and efficacy of gene therapy products. If you are interested in us, please feel free to contact us.
References
- El Andari J, et al. Production, Processing, and Characterization of Synthetic AAV Gene Therapy Vectors. Biotechnol J. 2021 Jan;16(1):e2000025.
Related Services


 Fig.2 Gene therapy manufacturing process. (CD Formulation)
Fig.2 Gene therapy manufacturing process. (CD Formulation)