Oral Thin Film Quality & Analytical Technologies
Inquiry
The oral thin film is a polymeric film intended to deliver therapeutic agents either locally or systemically in the oral cavity or through gastrointestinal absorption. It is a promising drug delivery system with advantages such as convenience, rapid release, and improved bioavailability. To ensure the quality and performance of oral films, CD Formulation offers various analytical techniques throughout their development, manufacturing, and quality control processes.
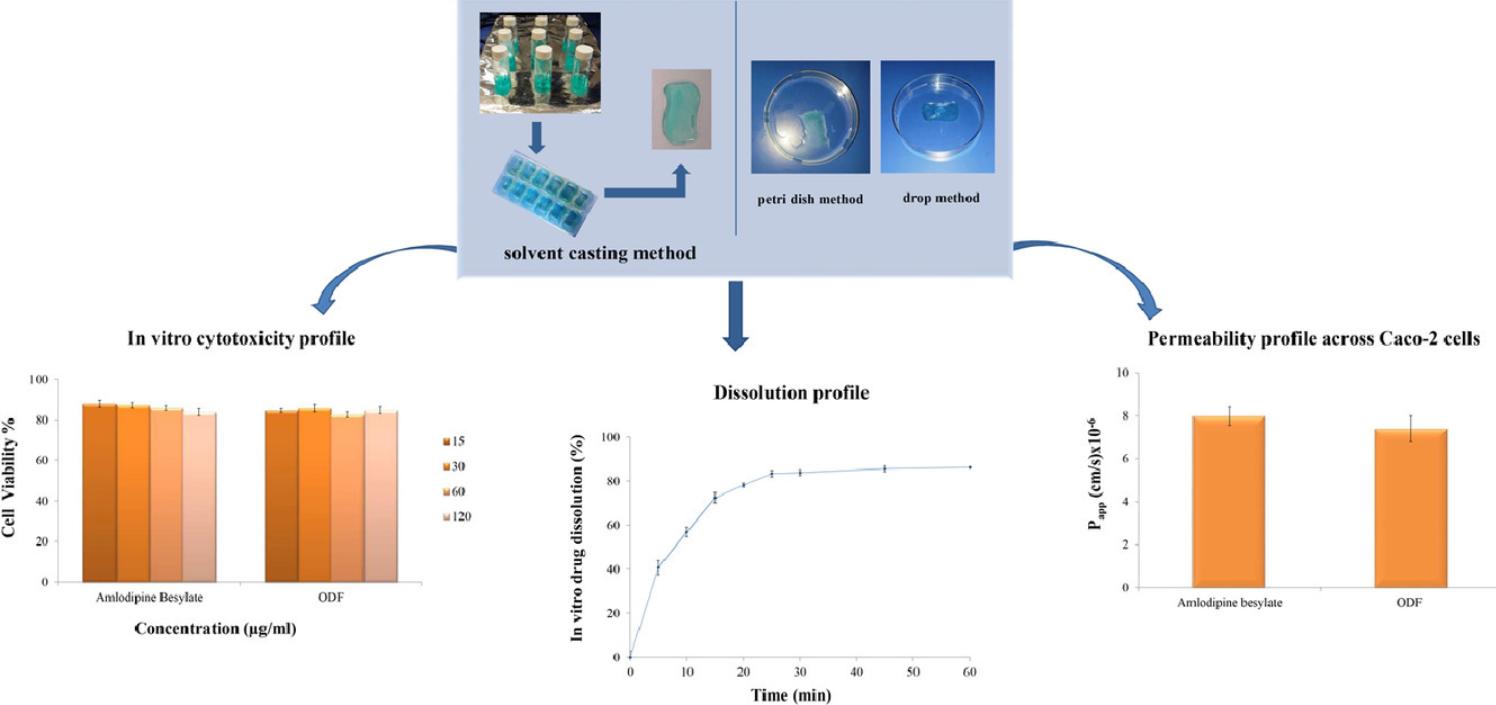 Fig.1 Characterization of pullulan-based orally disintegrating films. (Esra Pezik, et al.,2021)
Fig.1 Characterization of pullulan-based orally disintegrating films. (Esra Pezik, et al.,2021)
The Importance of Oral Thin Film Quality & Analytical Technologies
Quality & analytical technologies are essential for evaluating the physicochemical properties, chemical composition, and performance characteristics of oral thin film products, and this includes identification and quantification of active pharmaceutical ingredients and excipients, testing for impurities or degradation products, evaluation of batch-to-batch consistency, understanding drug release kinetics and bioavailability, product safety assessment, etc.
Our Oral Thin Film Quality & Analytical Technologies
We have professional oral thin film analysis technicians who use our quality & analysis technologies to help customers optimize the formulation, manufacturing process and quality control of oral thin film. We ensure that the oral thin film drugs produced are safe and effective, providing effective treatment results for patients. Our quality & analysis technologies include:
Our quality support is designed to ensure the safety, efficacy and compliance of oral thin film drug systems, providing comprehensive quality control for the development, manufacturing and distribution stages of oral thin film. Our oral thin film quality support capabilities include but are not limited to analytical method development, quality control testing, stability studies, manufacturing process support, oral thin film product development and process improvement, such as Quality by Design (QbD), risk assessments, method transfer and optimization, etc.
Analytical testing involves various test methods and techniques designed to evaluate the physical and chemical properties, mechanical properties, etc., of oral thin films, and it is essential to ensure the quality, performance and safety of oral thin film drug delivery systems. Our oral thin film quality support capabilities include but are not limited to physical characterization, chemical analysis, thermal analysis, in vitro dissolution testing, quantitative analysis, method validation, verification, etc.
Explore Our Quality & Analytical Technologies Services
CD Formulation pays excellent attention to oral thin film's quality and analytical aspects. With our advanced quality control platforms, we can provide our customers with quality control and analytical testing services for formulation development of oral thin film. Our analytical team performs in-depth quality control studies to ensure the quality of oral thin film in commercial production and clinical applications.
Formulation Development Support
Our oral thin film specialists offer assistance in developing and optimizing oral thin formulations, including selecting APIs and excipients, etc., to ensure product stability, efficacy, and patient acceptability.
Oral Thin Film Characterization
Relying on the advanced development platform and professional technical teams, we provide physicochemical and biological characterization services for oral thin film and provide support for quality control of oral thin film.
Stability Testing
Our services include conducting stability studies to assess oral thin film's shelf-life and storage conditions, ensuring product quality is maintained over time.
Preparation Method Screening and Optimization
Our technical team is aware of the advantages and limitations of each method. We will select the most suitable preparation method for oral thin films according to the physical and chemical properties of the APIs and optimize the chosen method.
Advantages of Our Oral Thin Film Quality & Analytical Technologies Services
- We introduced the QbD design method, which is a methodology used to build quality into products, and the QbD approach ensures oral thin-film drug development, ultimately scientifically understanding how process parameters affect product performance.
- We utilize cutting-edge technologies and equipment to develop and analyze oral thin film products, such as DSC, TGA, FT-IR, HPLC, HPLC-MS, etc., allowing for precise and accurate results.
- Our team of scientists and researchers have extensive experience and expertise in oral thin film formulation development, analytical testing, and quality control, ensuring that our clients receive high-quality and reliable services.
Published Data
Technology: Retrospective Quality by Design
Journal: International Journal of Pharmaceutics
IF: 5.8
Published: 2017
Results: In this study, risk assessment tools were used to identify parameters affecting oral thin films' critical quality attributes, namely percent drug release and residual water content. The parameters room temperature, room relative humidity, drying temperature and mixing equipment were used in the statistical modeling of the available data. The estimated models were then used to define the feasible working region. Statistical modeling indicates that the initial residual water content of the oral thin films is mainly affected by 2nd order interactions of room temperature, room relative humidity and drying temperature, while the stability of the drug release profile is mostly influenced by room temperature and an interaction between room relative humidity and drying temperature. This work shows that it is possible to apply rQbD to achieve a greater understanding of the manufacturing process of oral thin films and to define a proper design space.
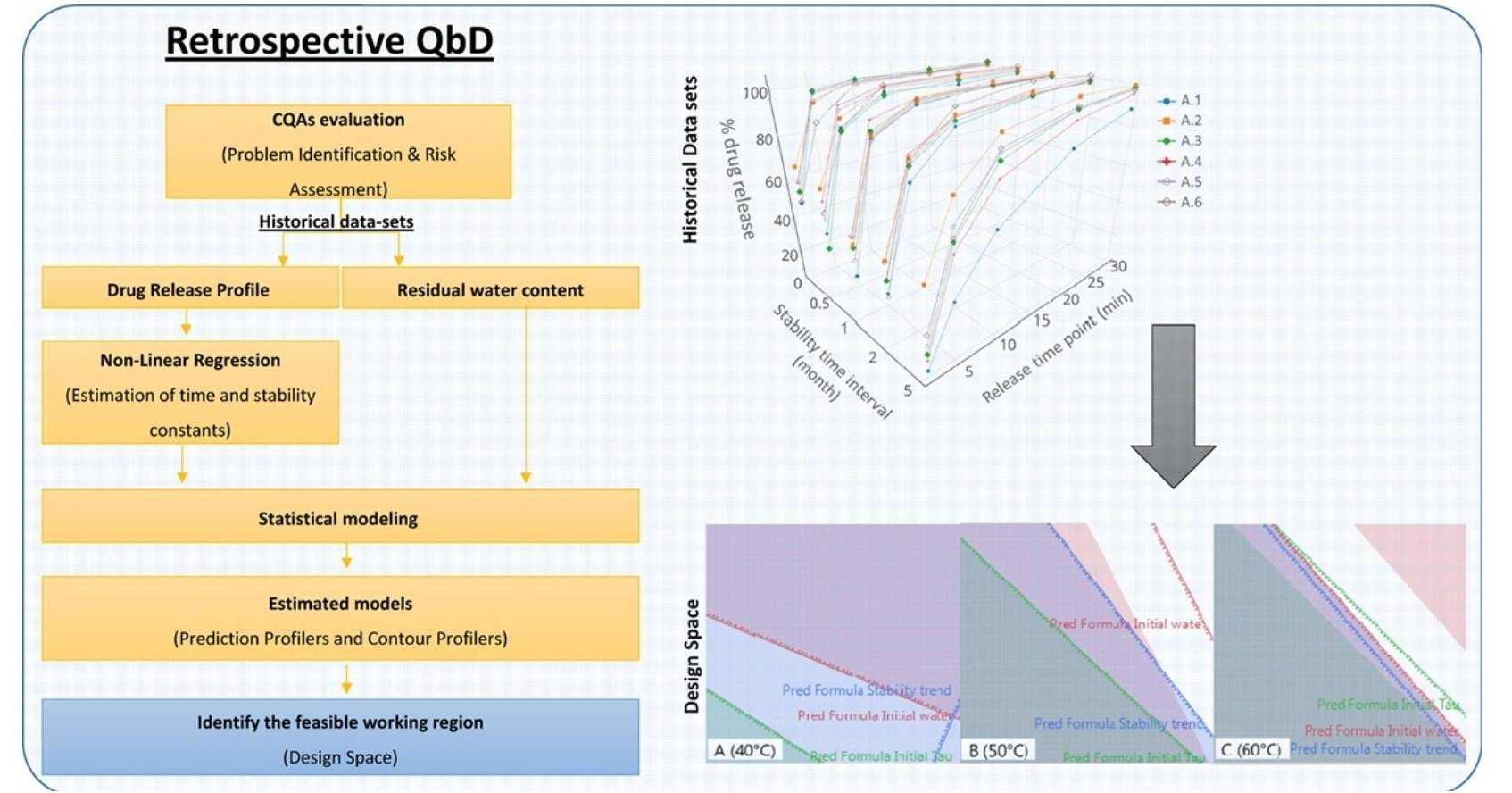 Fig.2 Retrospective Quality by Design (rQbD) applied to the optimization of orodispersible films.
Fig.2 Retrospective Quality by Design (rQbD) applied to the optimization of orodispersible films.
(Branca M.A. Silva, et al., 2017)
CD Formulation is committed to providing accurate, reliable, and actionable data that supports our clients in achieving oral thin film product development and quality objectives. If you require our oral thin film quality & analytical technologies services, please contact us by phone or email, and our colleagues will get back to you within three working days.
References
-
Esra Pezik, Tugba Gulsun, et al. Development and characterization of pullulan-based orally disintegrating films containing amlodipine besylate. European Journal of Pharmaceutical Sciences 2021, Vol (156).
- Branca M.A. Silva, Sílvia Vicente, et al. Retrospective Quality by Design (rQbD) applied to the optimization of orodispersible films. International Journal of Pharmaceutics. 2017,Vol(528):655-663.
How It Works
STEP 2
We'll email you to provide your quote and confirm order details if applicable.
STEP 3
Execute the project with real-time communication, and deliver the final report promptly.
Related Services


 Fig.1 Characterization of pullulan-based orally disintegrating films. (Esra Pezik, et al.,2021)
Fig.1 Characterization of pullulan-based orally disintegrating films. (Esra Pezik, et al.,2021) Fig.2 Retrospective Quality by Design (rQbD) applied to the optimization of orodispersible films.
Fig.2 Retrospective Quality by Design (rQbD) applied to the optimization of orodispersible films.
