Oral Thin Film Analytical Testing Capabilities
Inquiry
Rigorous, effective analytical testing is key in the oral thin film drug development and manufacturing lifecycle because this testing is used to verify control of drug chemistry. It can help identify barriers that prevent movement to the next phase in the development process. That's why it's critical to have efficient, accurate and high-quality analytical support for your oral thin film testing requirements. CD Formulation helps you design, develop, implement and manage an effective, safe and secure analytical testing program for an oral thin film product.
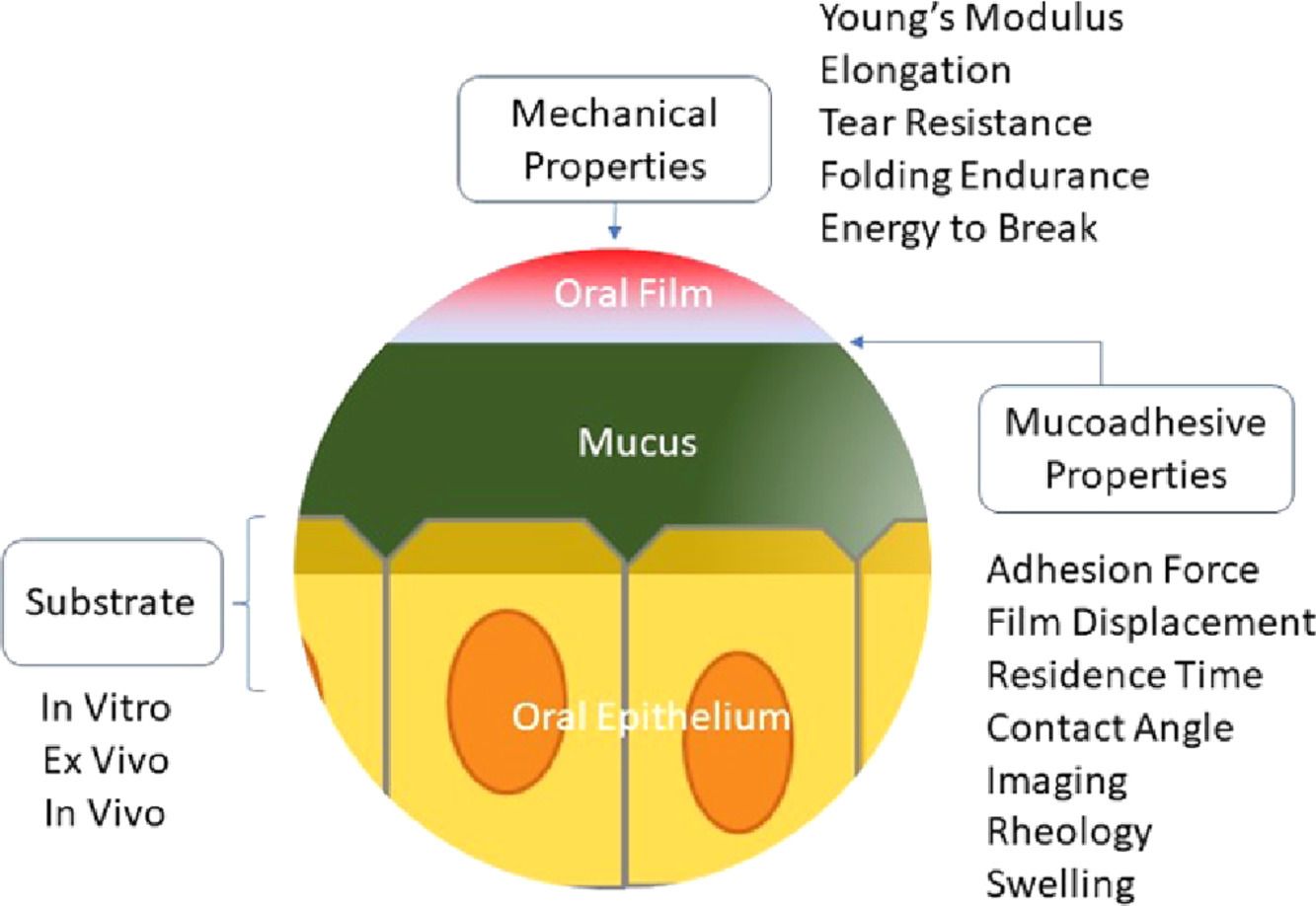 Fig.1 Characterization of oral films. (Samaneh Alaei, et al., 2021)
Fig.1 Characterization of oral films. (Samaneh Alaei, et al., 2021)
Our Oral Thin Film Analytical Testing Capabilities
Our oral thin film analytical testing involves analyzing various properties and attributes of the thin film formulation to ensure its quality, performance, and suitability for drug delivery.
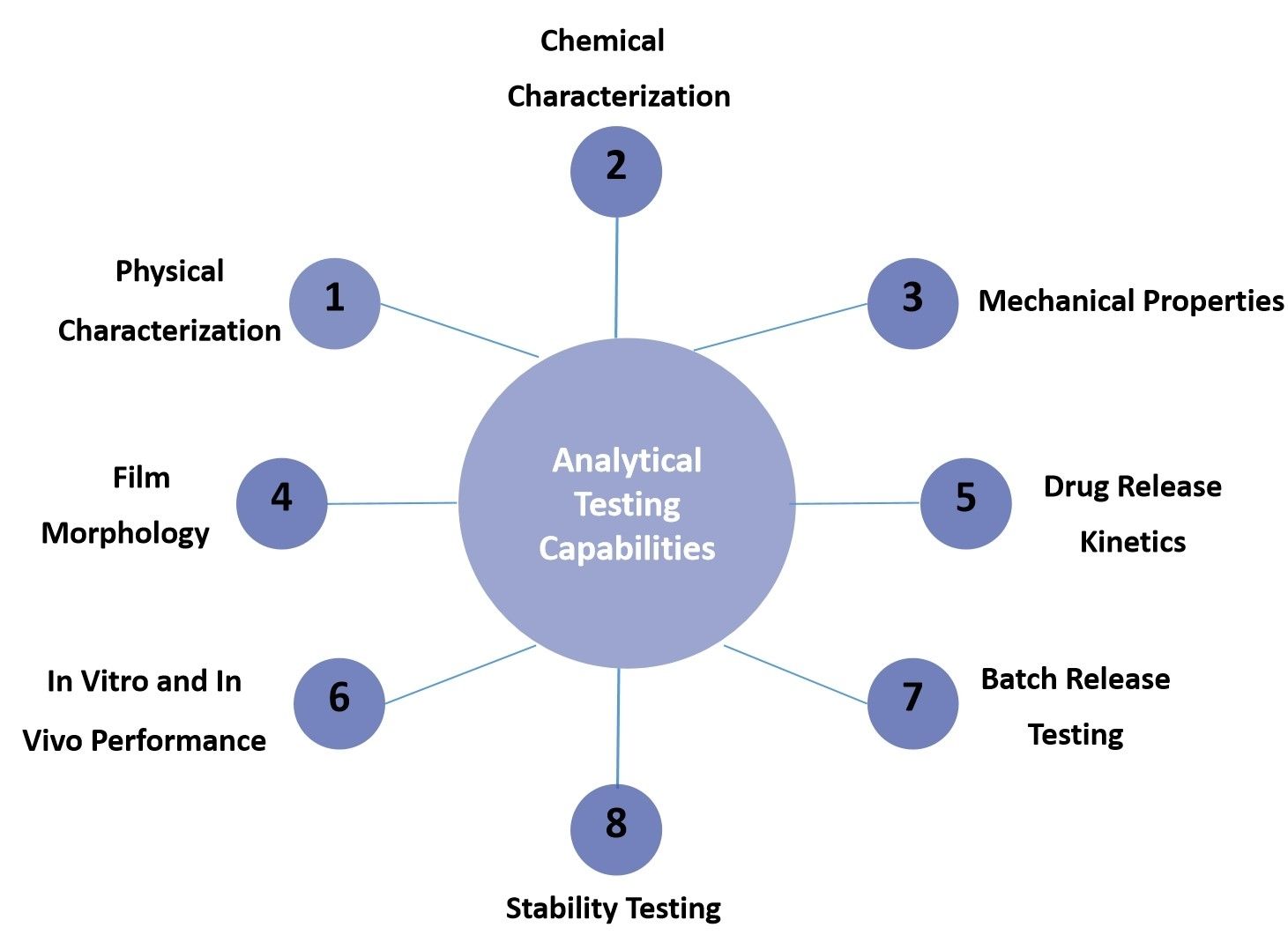 Fig.2 Our Oral Thin Film Analytical Testing Capabilities. (CD Formulation)
Fig.2 Our Oral Thin Film Analytical Testing Capabilities. (CD Formulation)
Physical Characterization
Physical characterization of the thin film includes organoleptic, thickness, weight, flexibility, and surface pH, which can impact the disintegration and dissolution of the thin film, as well as its administration.
Chemical Characterization
We usually use techniques such as chromatography and spectroscopy to perform oral thin film chemical characterization. This includes analyzing the composition of the thin film, including active pharmaceutical ingredients, excipients, and any potential impurities or degradation products.
Mechanical Properties
We have professional mechanical performance evaluation tools, evaluating mechanical properties such as tensile strength, elongation at break, adhesion strength, etc., which can provide insight into the film's robustness, durability, and ability to adhere to the oral mucosa.
Film Morphology
Analyzing the microstructure and surface morphology of the thin film can help understand its uniformity, smoothness, and potential interactions with the oral cavity.
Drug Release Kinetics
Assessing the release profile of the active ingredients from the oral thin film is critical for understanding its drug delivery behavior and ensuring the desired pharmacokinetic profile.
In Vitro and In Vivo Performance
Characterizing the in vitro dissolution behavior and in vivo performance of the oral thin film can provide insight into its bioavailability, onset of action, and overall therapeutic effectiveness.
Batch Release Testing
We provide our customers with batch release testing for raw materials, active pharmaceutical ingredients (APIs), and final products, including but not limited to assay, impurities, dissolution, content uniformity, stability, and microbial contamination testing.
Stability Testing
We established a unique and professional oral thin film stability testing strategy based on ICH guidelines and our analytical platform, evaluating the stability of oral thin film under various storage conditions.
Our Oral Thin Film Analytical Testing Techniques
- Chromatography: UHPLC, GC, IC, SFC
- Detection: UV, DAD, ELSD, CAD, FID
- Mass Spectrometry: LC-MS, LC-MS/MS (Q-TOF, TQ), GC-MS, GC-MS/MS
- NMR: 1H-NMR, 13C-NMR, multinuclear NMR, 2D-NMR
- Structural Identification: NMR, HRMS, FT-IR, UV
- Solid-state Characterization: XRPD, DSC, TGA, DVS, PSD, microscopy
- Mechanical Properties Characterization: DVS, Elmendorf tear thwing albert, UTMs, Texture Analyzers, Graves Tear Testers, Film Tensile Testers.
- General Testing: KF, potentiometric titrator, ROI, polarimeter
- Elemental Analysis: ICP-OES, IPC-MS
- ICH stability and photo-stability chambers
Our Workflow of Oral Thin Film Analytical Testing
We have a professional analysis and testing team that can quickly respond to your testing requirements, and we can analyze any testing during the development of oral thin film. Our testing process is as follows:
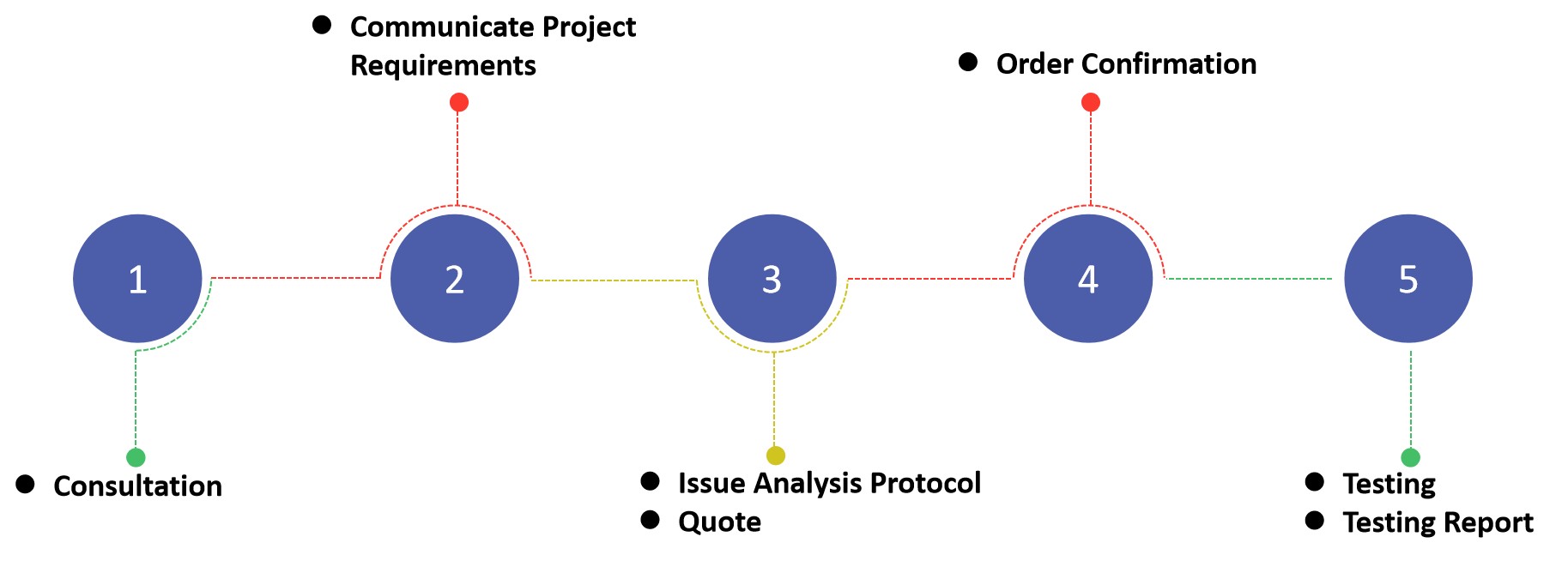 Fig.3 The Workflow of Oral Thin Film Analytical Testing. (CD Formulation)
Fig.3 The Workflow of Oral Thin Film Analytical Testing. (CD Formulation)
- Consultation: Discuss clients' specific requirements and issue detailed experimental protocols.
- Quote: Provide the customer with a quotation with a testing plan.
- Testing: Our expert analysts began testing and collecting data.
- Report: Based on the collected data, we will analyze, evaluate, and offer a complete experimental report.
Advantages of Our Oral Thin Film Analytical Testing
- Our experienced team performs tests using industry-standard processes, guaranteeing consistent testing results backed by experience and expertise.
- Our robust list of testing methods includes thermoanalytical analysis, GC-MS, SEM, X-ray fluorescence, FTIR, ICP-MS and more.
- As an ISO/IEC 17025 accredited laboratory, we have the highest technical competence, traceability and customer confidentiality standards.
Explore Our Oral Thin Film Analytical Testing
We offer a comprehensive range of analytical testing services for oral thin films. These services are designed to ensure oral thin film quality, safety, and efficacy throughout the product development and manufacturing process.
Physical and Chemical Analysis
Our professional analysis and testing technology runs through the entire development and production process of oral thin film, providing quality assurance for oral thin film products.
In Vitro Study
We provide professional in vitro disintegration and in vitro dissolution testing methods to evaluate the disintegration time and release of active ingredients of oral thin films, providing a basis for the formulation performance and formulation performance release kinetics and oral thin films.
Our comprehensive analytical testing helps you understand the development of your oral thin film and ensure it meets industry standards. If you require our oral thin film analytical testing services, please contact us by phone or email, and our colleagues will get back to you within three working days.
References
- Samaneh Alaei, Hamid Omidian. Mucoadhesion and Mechanical Assessment of Oral Films. European Journal of Pharmaceutical Sciences. 2021, Vol (159).
How It Works
STEP 2
We'll email you to provide your quote and confirm order details if applicable.
STEP 3
Execute the project with real-time communication, and deliver the final report promptly.
Related Services


 Fig.1 Characterization of oral films. (Samaneh Alaei, et al., 2021)
Fig.1 Characterization of oral films. (Samaneh Alaei, et al., 2021) Fig.2 Our Oral Thin Film Analytical Testing Capabilities. (CD Formulation)
Fig.2 Our Oral Thin Film Analytical Testing Capabilities. (CD Formulation) Fig.3 The Workflow of Oral Thin Film Analytical Testing. (CD Formulation)
Fig.3 The Workflow of Oral Thin Film Analytical Testing. (CD Formulation)
