Analytical Method Development and Validation for Oral Thin Film
Inquiry

Oral thin films are becoming more and more popular in oral administration. Therefore, in the development of oral thin films, we should attach great importance to the analytical method development and validation. Analytical method development requires an in-depth understanding for the product's key quality attributes (CQA), which is typically achieved by the implementation of analytical quality by design (AQbD). Method validation requires long-term monitoring of the method, including monitoring as changes occur, such as method transfer or production changes. Implementing new technologies often allows for more improvements, but the impact on legacy methods must be minimal and not interfere with monitoring of CQAs.
The Importance of Analytical Method Development and Validation for Oral Thin Film
Analytical method is a tool and means to reveal the quality of drug products, and method validation is a process to judge whether the analytical method is scientific and feasible. The analytical method is one of the important steps in the development of oral thin films, and its development and verification are very important for the quality control of oral thin films products and the guarantee of therapeutic effect. CD Formulation has experienced experts in analytical method development and validation and provides cGMP-based analytical method development and validation services for your oral thin films development according to industry standards and regulatory guidelines.
Our Analytical Method Development and Validation for Oral Thin Film Services
CD Formulation complies with internationally recognized quality management standards and provides standardized analysis and validation procedures for the development of your oral thin film delivery systems. Our analytical method development and validation for oral Thin Film services include but are not limited to:
Oral Thin Film Analytical Method Development and Optimization
Developing and optimizing reliable drug analysis methods is critical to ensuring the quality, purity, and stability of APIs and formulations. With our deep expertise and extensive analytical method development for oral thin films drugs, CD Formulation provide you customized assay method development and optimization services to support your analytical needs.
Oral Thin Film Analytical Method Validation
Analytical method validation ensures that the method is reliable in normal use and is accurate, specific, and precise within the specified range for analysis. Sometimes called "the process of providing documented evidence that the method achieves its intended purpose." CD Formulation develops standardized validation procedures based on oral thin films.
Oral Thin Film Analytical Method Transfer
Formal transfer of analytical methods is an essential process in oral thin films development. This process is to ensure that the established analytical method performs as expected in the receiving laboratory. CD Formulation provides cGMP-based analytical method transfer services and adheres to internationally recognized quality management standards to ensure the correctness and reliability of analytical methods.
Oral Thin Film Pharmaceutical Deformulation Service
Pharmaceutical deformulation, which is widely used in the pharmaceutical industry, is the process of analyzing any individual ingredient in a drug to determine and understand its composition, formulation, and manufacturing. CD Formulation’s oral thin films pharmaceutical deformulation services are designed to meet our clients’ generic drug development needs, provides formulation evaluation data for Q1/Q2 and proves similarity and bioequivalence to the reference preparation, thereby helping you shorten development time and speed up the time to market.
What Problem Can We Solve?
- Reverse Analysis of Oral Thin Films: The complete composition analysis of the existing oral thin films was carried out.
- Identification of Target Components: Our team of experts communicates with the client to determine the oral thin films for analysis, as well as the specific goals and requirements required for the analysis method.
- Selection of Analytical Method and System: According to the analysis of the project, select the appropriate detection instrument, analysis method and system.
- Selection of Initial Conditions and Separation: The most appropriate separation mode is selected based on the solubility of the sample and the differences between the target analyte and other compounds or substrates in the sample.
- Verification of Method: Conduct thorough method suitability tests to confirm the suitability of the method, including accuracy, precision, reproducibility, repeatability, specificity and selectivity, limit of detection (LOD), limit of quantification (LOQ), linearity, range, stability.
- Transfer of Method: Develop a comprehensive transfer scheme to confirm the equivalence of transfer methods.
Our Advantages in Analytical Method Development and Validation for Oral Thin Film
- New method Development: We are very experienced in developing methods where there are no standards.
- Analytical Characteristics: Typical analytical characteristics are used, including accuracy, precision, specificity, LOD/LOQ, linearity, range, and robustness.
- Flexible: Our analytical laboratories offer flexible processes for more complex and/or technology-based methods.
- Strict: All our work processes strictly comply with cGMP requirements.
- Timeliness: We provide fast turnaround time for method feasibility assessment, protocol execution, and final report generation.
Published Data
Technology: Quantification Method Development and Validation of the Pregabalin and Methylcobalamin.
Journal: Gels
IF: 4.6
Published: 2023
Results: In this study, the quantification method was developed and validated by making modifications on the simultaneous determination methods of PG and MC. With the HPLC quantification method, PG and MC were analyzed in the presence of components forming OTF formulations, and the originality of the method was proven.
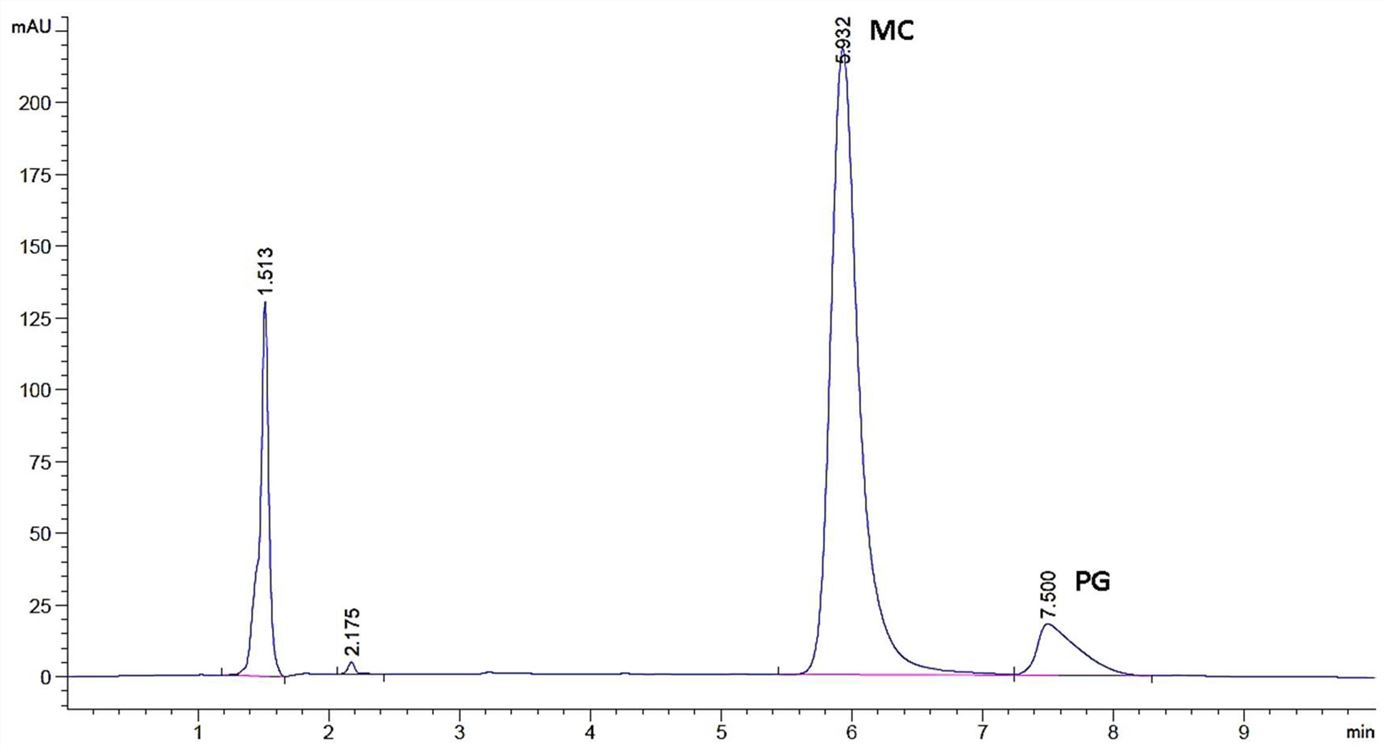 Fig.1 Chromatogram of PG and MC. ((Emrah Özakar, et al., 2023)
Fig.1 Chromatogram of PG and MC. ((Emrah Özakar, et al., 2023)
CD Formulation provide expertise in analytical method development and validation of oral thin films and provides comprehensive technical support for the development of oral thin films as well as the launch and stability testing of commercial products. If you have a requirement about our oral thin film analytical method development and validation services, please contact us by phone or email, our colleagues will reply to you within three working days.
References
- Bengi Uslu, Henk Lingeman, et al. Analytical Method Development and Validation of Pharmaceutical Analysis Using Chromatographic Techniques. Editorial. 2012.
- Emrah Özakar, Rukiye Sevinç-Özakar, et al. Preparation, Characterization, and Evaluation of Cytotoxicity of Fast Dissolving Hydrogel Based Oral Thin Films Containing Pregabalin and Methylcobalamin. 2023, 9(2).
How It Works
STEP 2
We'll email you to provide your quote and confirm order details if applicable.
STEP 3
Execute the project with real-time communication, and deliver the final report promptly.
Related Services


 Fig.1 Chromatogram of PG and MC. ((Emrah Özakar, et al., 2023)
Fig.1 Chromatogram of PG and MC. ((Emrah Özakar, et al., 2023)
