Preparation Method Screening of Oral Thin Films
Inquiry

As an oral drug delivery system, oral thin films have developed over the years and have unique advantages in some disease areas, such as schizophrenia, Alzheimer's disease, antibacterial, anti-inflammatory and allergy, hypertension, and anti-thrombosis. The quality of the oral thin films is particularly important to the treatment of the disease. Many factors affect the quality of the oral thin films. Among them, the preparation method is the second important factor that affects the quality of oral thin films. The preparation methods of oral thin films include solvent casting method, hot melt extrusion method, semi-solid casting method, 3D printing method, etc., but each method has its advantages and limitations.
CD Formulation is experienced in the screening of preparation methods for oral thin films. Our technical team is aware of the advantages and limitations of each method, we will help customers select the most suitable preparation method for oral thin films according to the physical and chemical properties of the APIs.
Our Technologies for Oral Thin Film Preparation
CD Formulation has advanced technology platforms for oral thin film preparation, and we can provide a variety of preparation technologies to support your oral film development.
Solvent Casting Technology
Solvent casting is the most used preparation technology. The process is to dissolve or suspend film-forming materials, raw materials, and other auxiliary materials in suitable solvents, such as water, ethanol, acetone, etc. The air bubbles are removed after mixing evenly, and then cast on the mold or coated with film forming agent and cut and package after drying. This technology method may has some problems, such as the solvent cannot be completely dried, the film surface is rough, the film thickness is inconsistent and the finished product stability is poor.
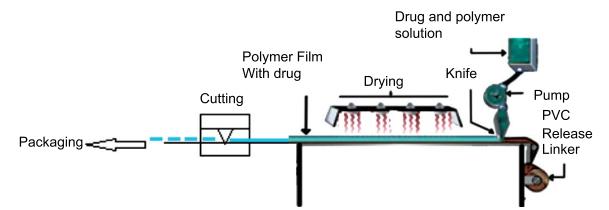 Fig.1 Commercial manufacturing process of solvent casting. (Suhani Sinha, et al., 2019)
Fig.1 Commercial manufacturing process of solvent casting. (Suhani Sinha, et al., 2019)
Hot Melt Extrusion Technology
Hot melt extrusion, which does not require the use of solvents during the production process, shapes a mixture of polymers, drugs, and other excipients into a film by melting all the components. This technology is often used to prepare solvent-free oral thin films. Compared with solvent casting, this technology method is solvent-free and does not require a drying process, but the APIs and other components used have high temperature resistance requirements. The melting process in hot melt extrusion may have an impact on the stability of the APIs, taste, and stability of the polymer.
3D printing Technology
3D printing technology combines the advantages of printing and solution casting, and its advantages are good stability, accurate drug loading, more flexible, and conducive to personalized drug delivery, etc. Compared with other technologies, this technology is not suitable for high-throughput industrial production.
Semi-solid Casting Technology
This technique typically mixes a water-soluble film-forming polymer solution with an acid-insoluble polymer solution to form a uniform viscous solution (such as cellulose acetate phthalate and cellulose acetate butyrate), after ultrasonic treatment, it is coated on an untreated cast film.
Rolling Technology
In rolling technology, a solution or suspension containing a drug is rolled onto a carrier. The solvent used is mainly water or a mixture of water and alcohol. The film is dried on the rollers and then cut into the desired shapes.
Our Workflow of Screening Oral Thin Film Preparation Methods
- Customer requirement creation
- Signing a confidentiality agreement
- Analysis and evaluation of physical and chemical properties of APIs and excipient ingredients.
- Study on compatibility of APIs and excipient ingredients.
- According to the above research results, screening preparation method.
- Determine the reasonableness of the preparation method
- Submit a complete method screening evaluation report
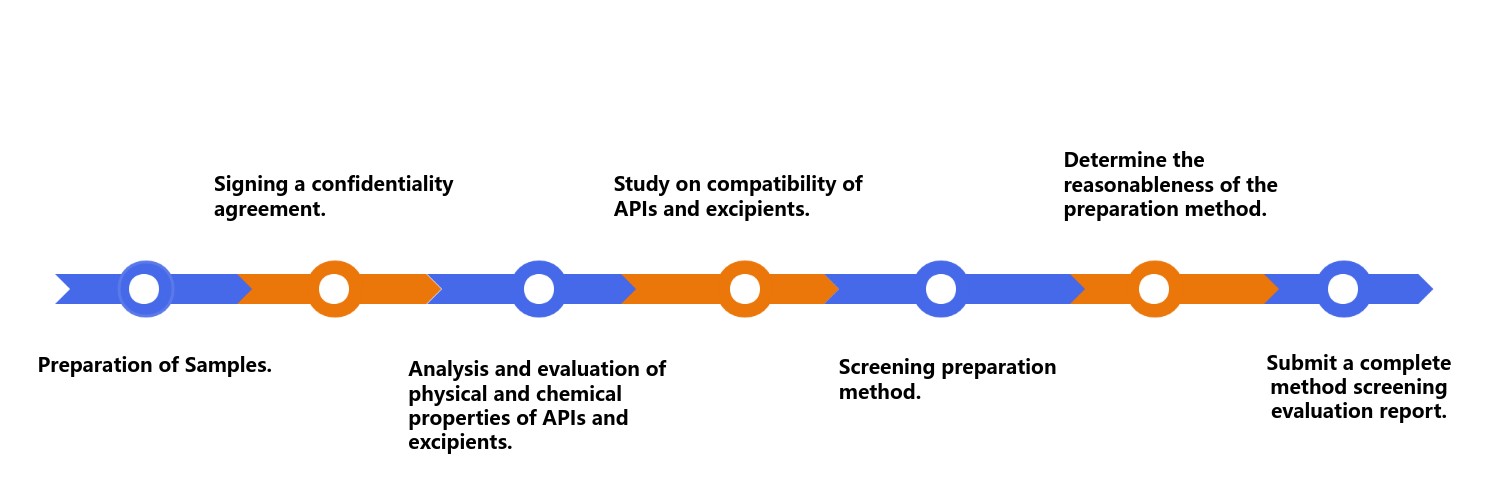 Fig.2 The Workflow of Screening Oral Thin Film Preparation Methods. (CD Formulation)
Fig.2 The Workflow of Screening Oral Thin Film Preparation Methods. (CD Formulation)
Our Advantages in Screening Oral Thin Film Preparation Methods
- State-of-the-Art Technology Platform: We have state-of-the-art technology platform for the preparation of oral thin films, we will be based on customer needs, the properties of raw materials and costs to select the most suitable preparation method.
- One-stop Services: We offer one-stop services from raw material property assessment, formulation development, preparation method screening, preparation method transfer and commercial production.
- Complete Documentation: We provide a complete preparation method screening evaluation report.
Published Data
Technology: Solvent Casting Technique
Journal: Human Journals
IF: 9.8
Published: 2023
Results: This study aims to create oral thin films of Ondansetron utilizing the solvent casting process. Oral thin patches were created by combining different super disintegrants such as ludiflash (1,2,3,4 and 5% w/w), crospovidone (1,2,3,4 and 5% w/w) concentrations with Gelatin and Polyvinyl alcohol as film-forming agents.
 Fig.3 Solvent Casting Technique. (Dhendi Sandhya, et al., 2023)
Fig.3 Solvent Casting Technique. (Dhendi Sandhya, et al., 2023)
CD Formulation uses professional knowledge and rich experience in the development of oral thin films to provide customers with reliable preparation method screening services, our team is composed of experienced chemical analysts and oral thin films preparation experts, with advanced oral thin films preparation technology platform. If you have a requirement about our preparation method screening of oral thin film services, please contact us by phone or email, our colleagues will reply to you within three working days.
References
- Suhani Sinha, Rohit Dutt. Oral Soluble Films: Attributes of the Polymeric Material and Critical Process Parameters for Designing Good Quality Films. Current Applied Polymer Science. 2019, Vol (3): 167-188.
- Muhammad Irfan, Sumeira Rabel, et al. Orally disintegrating films: A modern expansion in drug delivery system. Saudi Pharmaceutical Journal. 2016, Vol (24): 537-546.
- Dhendi Sandhya, P. Jyothi. Formulation and In Vitro Evaluation of Ondansetron Oral Thin Films. Human Journals. 2023, Vol (26):301-314.
How It Works
STEP 2
We'll email you to provide your quote and confirm order details if applicable.
STEP 3
Execute the project with real-time communication, and deliver the final report promptly.
Related Services



 Fig.1 Commercial manufacturing process of solvent casting. (Suhani Sinha, et al., 2019)
Fig.1 Commercial manufacturing process of solvent casting. (Suhani Sinha, et al., 2019) Fig.2 The Workflow of Screening Oral Thin Film Preparation Methods. (CD Formulation)
Fig.2 The Workflow of Screening Oral Thin Film Preparation Methods. (CD Formulation) Fig.3 Solvent Casting Technique. (Dhendi Sandhya, et al., 2023)
Fig.3 Solvent Casting Technique. (Dhendi Sandhya, et al., 2023)
