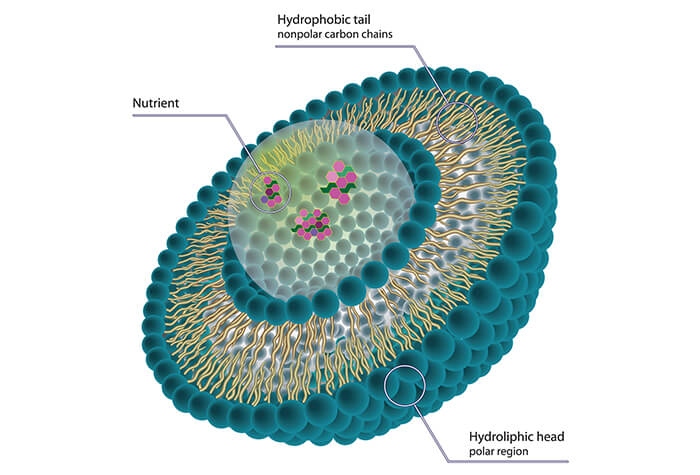
Nanomedicine - CD Formulation
Call Us:
- Home
- Services
- Nanoformulation Development
- Nanoparticle Development for Nanomedicine
- Solid Lipid Nanoparticle Development for Nanomedicine
- Albumin Nanoparticle Development for Nanomedicine
- Lipid Nanoparticle Development for Nanomedicine
- Polymeric Nanoparticle Development for Nanomedicine
- Biomimetic Nanoparticle Development for Nanomedicine
- Environmentally Responsive Nanoparticle Development for Nanomedicine
- Nanosuspension Development for Nanomedicine
- Liposome Development for Nanomedicine
- Exosome Development for Nanomedicine
- Nanostructured Lipid Carrier Development for Nanomedicine
- Nanoemulsion Development for Nanomedicine
- Nanocrystal Development for Nanomedicine
- Polymeric Micelle Development for Nanomedicine
- Nanofiber Development for Nanomedicine
- Nanocapsule Development for Nanomedicine
- Nanogel Development for Nanomedicine
- Microparticle Development for Nanomedicine
- Targeting Nanoformulation Development
- Generic Nanoformulation Development
- Oral Nanoformulation Development
- Nanoparticle Development for Nanomedicine
- Nanoformulation Analysis
- Characterization of APIs and Nanocarriers
- Nanoformulation Mean Particle Size Distribution and Testing Service
- Zeta Potential Measurement Service for Nanoformulations
- Nanostructure Characteristics Analytical Service
- Crystallinity Testing Service for Nanoformulations
- Microscopic Morphology Testing of Nanoparticles
- Nanoformulation Surface Property Testing Service
- Nanoformulation Molecular Weight Distribution Testing Service
- Nanoformulation Encapsulation Efficiency Testing Service
- Nanoformulation Drug Loading Testing Service
- Nanoparticle Concentration Testing Service
- Drug Release Testing Service for Nanocarriers
- Nanoformulation Thermal Performance Analysis
- Nanoformulation Critical Micelle Concentration Testing Service
- Nanoformulation Critical Aggregation Concentration Testing Service
- Nanoformulation Elemental Impurity Testing Service
- Nanoformulation Stability Testing Service
- Nanoformulation Residual Solvent Testing Service
- In Vitro Release Determination of Nanoformulations
- Endotoxin and Sterility Testing Service of Injectable Nanoformulations
- Nanoformulation Analytical Method Development and Optimization
- Nanoformulation Analytical Method Validation and Transfer
- Nanoformulation Biological Evaluation
- Nanoformulation Batch Release Testing Services
- Nanoformulation Pilot Scale-up Services
- Nanoformulation Development
- Technologies
- Nanotechnology Platforms for Nanoformulations
- Nanotechnology for Drug Delivery
- Targeting Drug Delivery System for Nanoformulations
- Microparticle Drug Delivery System for Nanoformulations
- Colloidal Drug Delivery System for Nanoformulations
- Self-emulsifying Drug Delivery System for Nanoformulations
- Exosome Drug Delivery System for Nanoformulations
- In Vivo Drug Delivery Strategies Based on Nanotechnology
- Co-encapsulation Nanomaterial Drug Delivery System for Nanoformulations
- Selective Organ Targeting Nanoparticle Platform for Nanoformulations
- Nanoformulation Technology for Biologics
- Nanochip Technology for Nanoformulations
- Applications
- Order Online
- Company
- Inquiry