Conventional Technologies for Liposome Preparation
Inquiry
At CD Formulation, we leverage a deep understanding of manufacturing processes to develop innovative liposome products. Our expertise spans various conventional preparation techniques, ensuring reliable solutions that meet diverse customer needs. We offer comprehensive support, a state-of-the-art technology center, and the expertise needed to address challenges and deliver effective liposome formulations.
Key Conventional Approaches to Liposome Preparation
The primary objective of the liposome nanoparticle formation method is to produce monodisperse particles with a narrower size distribution, achieve effective drug encapsulation, and maintain the long-term colloidal stability of the product.
Conventional methods typically involve dissolving lipids in a volatile organic solvent, followed by mixing with an aqueous phase. The presence of the organic solvent may impact the chemical properties of the incorporated active compound or influence the stability (or toxicity) of the nanoparticles generated. The conventional approach to liposome preparation involves the following key stages:
- Lipid solubilization in organic solvents
- Evaporation of the resulting lipid solution from the organic solvent
- Hydration of the lipid with an aqueous medium (followed by stirring)
- Size reduction (and/or layering modification)
- Shaping and processing (purification, sterilization)
- Characterization of the final nanoformulation
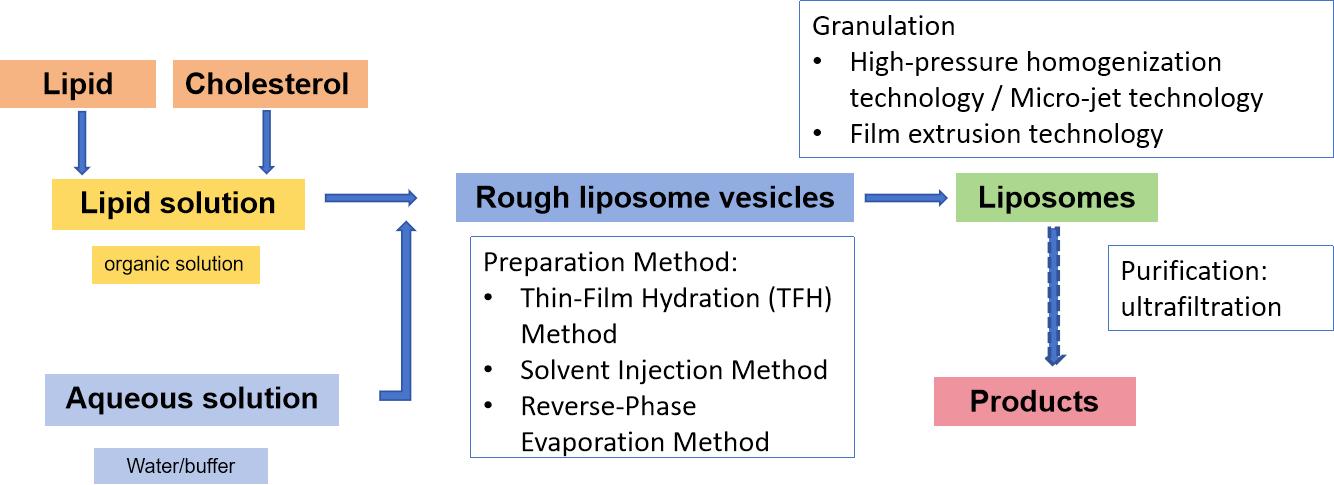 Fig.1 Flowchart of Liposome Formulation Preparation (without drug loading process). (CD Formulation)
Fig.1 Flowchart of Liposome Formulation Preparation (without drug loading process). (CD Formulation)
Our Conventional Liposome Preparation Technologies
The thin-film hydration method, also known as the Bangham method, is the oldest, most widely used, and simplest technique for preparing multivesicular liposomes. Through the TFH method, we have effectively employed liposome nanotechnology to encapsulate various lipophilic drug molecules (such as docetaxel (DTX), paclitaxel (PTX), quercetin, resveratrol (RES)) and various hydrophilic components (such as targeted proteins, small interfering RNA, and siRNA) for numerous clients.
Liposomal precursors have substantial potential for delivering drugs with low oral bioavailability. Leveraging our well-established platform and experienced team of scientists, we offer a diverse range of liposomal precursors for your selection. Furthermore, we can provide customized services and expand our formulations for drug delivery.
The ethanol solvent injection method is widely favored in the literature for efficiently producing small monolayer liposomes. We have developed expertise in this technology and continually optimized parameters to tailor the production process for generating uniform, small liposomes. Moreover, in gene delivery applications, our solvent injection liposomes are preferred over those prepared by thin film hydration due to their superior transfection efficiency.
In the process of liposome preparation, reverse vaporization represents a typical method. The lipid mixture is dispersed and dissolved in a solvent, followed by evaporation of the lipid solvent. Subsequently, the lipid membrane obtained after evaporation is redissolved in the organic phase.
The detergent removal method is a gentle process that can be utilized to generate various types of vesicles and highly uniform liposomes. This approach is based on the formation of detergent-lipid micelles, followed by the elimination of the detergent to yield liposomes. Based on our experience, the size and uniformity of liposomes are contingent upon the rate and extent of detergent removal, as well as the ratio of phospholipids to detergent.
Our Advanced Liposome Preparation Platforms
| Facilities and Techniques |
Specifics |
| Techniques for Eliminating Residual Solvents |
- Evaporation Method: The liposome is placed in a vacuum vaporizer to evaporate the organic solvent. The method is suitable for volatile organic solvents. In the evaporation method, the evaporation speed and temperature need to be controlled to ensure that the quality is not affected.
- Nitrogen Blowing Method: Nitrogen is passed through the bottom to remove a large amount of organic solvents in the system.
- Alternative Techniques: Solid-phase extraction and purification columns.
|
| Techniques for Liposome Size Control |
- Ultrasonic Technology: It generates highly concentrated and dense energy, making it suitable for controlling the size of various liposome samples (typically recommended for volumes less than 10 ml) in small quantities, particularly for regulating the size of drug-loaded liposomes in small batches.
- Shearing Technology: Suitable for some special passive drug-loaded liposomes, multi-vesicle liposomes, etc.
- Homogenization Technology: Essential for a variety of drug-loaded lipid emulsions.
- Extrusion Technology: Fully exploiting the structural and performance characteristics of liposome membrane materials, operating just above the phase transition temperature of phospholipids. It controls size and distribution with the shearing force of the membrane material.
|
| Drug Delivery Platform |
- This platform supports both active and passive drug delivery technologies. On this platform, we have successfully used liposomes to transport both small-molecule chemical drugs and large-molecule biological drugs, accumulating extensive R&D experience.
|
Advantages of Our Conventional Liposome Techniques
- Advanced Formulation and Optimization Services: We offer sophisticated techniques for formulating and optimizing liposome parameters. Our expertise lies in traditional liposome preparation, including method selection, formulation design, process optimization, and validation analysis. We are dedicated to continuous innovation and improvement to provide our clients with high-quality liposome products and to discover new application capabilities.
- State-of-the-Art Equipment for R&D and Production: Our research and production facilities are equipped with advanced equipment to cater to the evolving demands of the research and development process. Designed and built in compliance with GMP standards set by regulatory bodies such as the US FDA, EMA, and PMDA, our facilities are backed by a comprehensive cGMP quality management system.
- Expert Core Team for Liposome R&D : Our core team is composed of skilled professionals from leading biopharmaceutical companies, bringing their knowledge in chemistry, biotechnology, medicine, and lipid formulations to the table. With extensive experience in liposome research, quality management, and industrial production, our team is well-equipped to support researchers in their liposome development journey.
Explore Our Liposome Preparation Services
Liposome Customization Services
We can design and synthesize liposomes with tailored functions and properties to meet specific research needs, including drug delivery objectives, functionality, and size specifications.
Universal Liposome Characterization
The physicochemical properties of liposomes can influence the performance of liposomal drugs, encompassing aspects such as liposome morphology, surface characteristics, structure and integrity, charge distribution, drug encapsulation efficiency, phase transition temperature, particle size distribution, etc.
Liposome Formulation Process Development
This service assesses the impact of various factors—such as drug-to-fat ratio, preparation method, loading technique, buffer type and ratio, API dosage, and water-oil ratio—on liposome characteristics and functionality. By adjusting these parameters, we can refine the structural properties of liposomes.
As a leader in nanoparticles, CD Formulation specializes in providing professional liposome technology to customers and advancing the development of the liposome field. Please feel free to contact us if you need any assistance.
How It Works
STEP 2
We'll email you to provide your quote and confirm order details if applicable.
STEP 3
Execute the project with real-time communication, and deliver the final report promptly.
Related Services


 Fig.1 Flowchart of Liposome Formulation Preparation (without drug loading process). (CD Formulation)
Fig.1 Flowchart of Liposome Formulation Preparation (without drug loading process). (CD Formulation)
