Proliposome Method for Liposome Preparation
Inquiry
Proliposomes are produced by encapsulating phospholipid solutions into a sugar matrix using methods such as rotary evaporation, spray drying, or fluidized bed coating. Upon hydration, proliposomes transform into functional liposomes, offering improved stability and ease of handling. CD Formulation specializes in leveraging this innovative technique to support researchers and pharmaceutical development projects. Our expertise enables the production of high-quality proliposomes that address challenges associated with traditional water-soluble liposomes, such as aggregation and fusion.
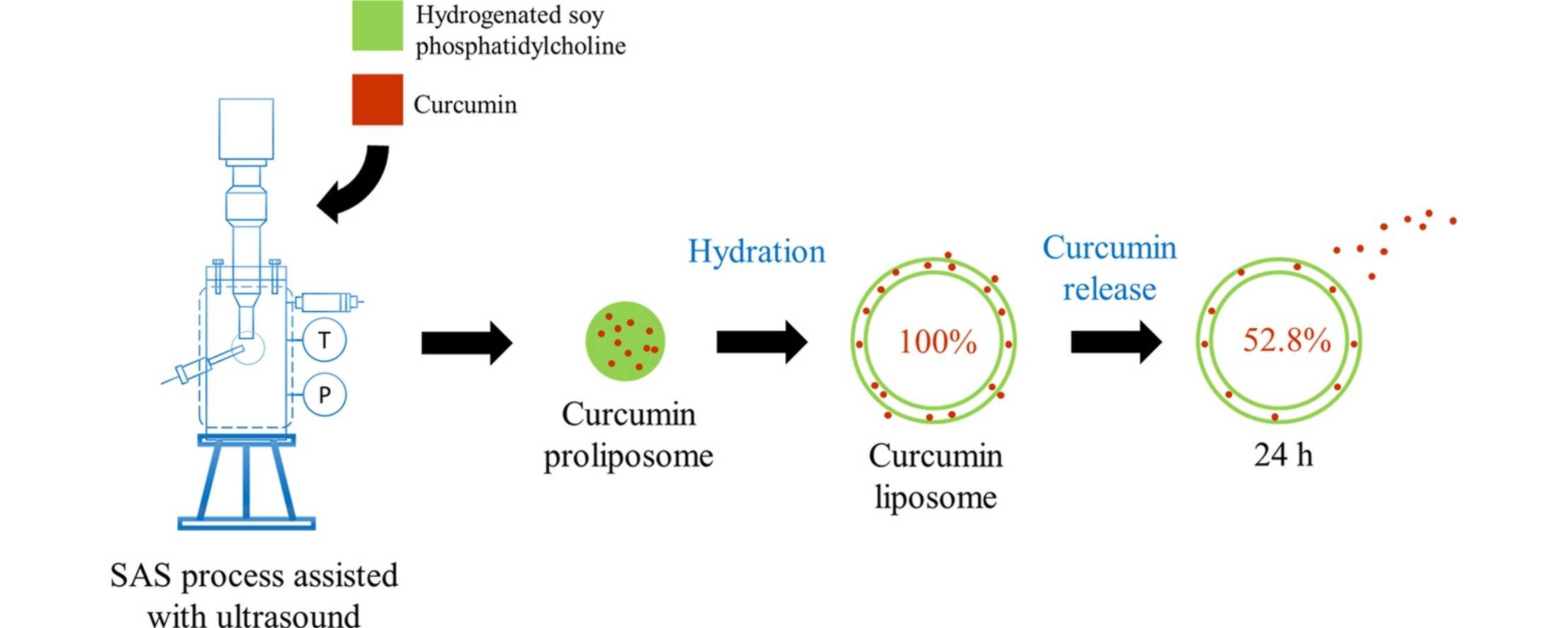 Fig.1 Illustration for Release-controlled curcumin proliposome. (Jia J, et al, 2016)
Fig.1 Illustration for Release-controlled curcumin proliposome. (Jia J, et al, 2016)
What is the Proliposome Method for Liposome Preparation?
The thin-film hydration method, commonly referred to as the Bangham method, is named after the British scholar who first identified liposomes, Bangham. This technique represents the most established approach for liposome preparation. The process entails dissolving phospholipids in an organic solvent, followed by evaporating the solvent under reduced pressure to yield a lipid-thin film. Subsequently, a suitable aqueous medium is introduced. At the phase transition temperature of the phospholipids, this lipid thin film hydrates and detaches, self-assembling into closed spherical structures known as liposomes. This method is straightforward and easily executed, achieving nearly 100% encapsulation efficiency for most lipophilic drugs. However, it tends to produce liposomes with a heterogeneous size distribution; thus, necessitating size control measures such as polycarbonate membrane extrusion or homogenization techniques. Furthermore, maintaining sterile conditions throughout the procedure is essential to ensure product sterility.
Our Proliposome Preparation Services
Screening Methods for Proliposome Customization
Due to diverse factors such as vesicle size, size distribution, encapsulation capacity, and retention of contents, it is necessary to select the appropriate method. We screen drugs based on their physicochemical properties, type of phospholipid required, target particle size range, and preparation method.
Proliposome Characterization
Primarily for the characterization of services including, but not limited to, particle size, zeta potential, encapsulation efficiency, drug loading, etc.
Hydration Study and Vesicle Formation
It is important to determine the formation of liposomal vesicles following hydration of the proliposomal formulation in vitro. We confirm the formation of vesicles with light microscopy: The liposome suspension should be applied onto a glass slide and air-dried at room temperature. Subsequently, the resulting dry film of the liposome suspension should be examined for vesicle formation.
In Vitro Release from Proliposomes
We conducted in vitro drug release studies of proliposomes using various techniques, such as USP dissolution apparatus Type I, Franz diffusion cells, dialysis tubes, reverse dialysis, glass paper diffusion membranes, transparent diffusion cells, and spectral molecular porous membrane tubes.
Our Capacities for Proliposome Method for Liposome Preparation
| Techniques and Platforms |
Specifics |
| Physicochemical Characterization |
Including but not limited to:
- particle size
- potential
- encapsulation rate
- drug loading
- morphology, etc
|
| Hydration Study and Vesicle Formation Platform |
- To determine the formation of liposomal vesicles following hydration of the proliposomal formulation.
- To confirm the formation with light microscopy.
|
Why Choose CD Formulation?
Advanced Proliposome Techniques
- Proficient in liposome thin-film hydration (TFH) preparation technique
- Formulation development capability
- Process optimization capability
- Size control capability
Powerful Proliposome Analysis Platforms
- Advanced platform supports the development and customization of multiple proliposomes, based on a various preparation processes and methods.
- Comprehensive analysis service. From physical and chemical properties, in vitro release studies, to in vivo evaluation, we guarantee to provide our customers with highly stable, effective, and less toxic side effects of the proliposomes.
A Great and experienced Team
- Successfully provided vital support to numerous corporations and academic institutions, enabling them to delve deeply into liposome thin-film hydration (TFH) preparation techniques.
- Our teams consist of many scientists with multi-disciplinary backgrounds who are well-versed in the development and evaluation of multiple proliposomes.
Published Data
Technology: modified thin film hydration (MTFH) technique
Journal: International Journal of Pharmaceutics
IF: 10.7
Published: 2022
Results: In this study, the authors aimed to develop stable and controlled-release curcumin precursor liposomes (CPL) utilizing ultrasound-assisted supercritical anti-solvent (SAS) technology. Given that the melting point of HSPC is elevated, no additional components are required, thereby streamlining the process of forming precursor liposomes. Consequently, this research employed HSPC as the wall material. The influence of various process parameters on CPL formation was systematically investigated, along with an assessment of curcumin stability and release from these liposomes. Operational parameters were optimized through response surface methodology (RSM). Ultrasound was implemented to enhance the SAS process for precise control over particle morphology. Furthermore, the impact of CPL morphology on drug release and stability was thoroughly examined.
CD Formulation provides customized proliposome preparation services to facilitate innovative lipid-based drug delivery systems. By addressing the inherent limitations of water-soluble liposomes, our solutions ensure enhanced formulation stability and efficiency, supporting a wide range of pharmaceutical R&D applications. Contact us for expert assistance with your proliposome preparation needs.
References
- Jia J, Song N, et al. Release-controlled curcumin proliposome produced by ultrasound-assisted supercritical antisolvent method. The Journal of Supercritical Fluids. 2016 Jul 1;113:150-7.
How It Works
STEP 2
We'll email you to provide your quote and confirm order details if applicable.
STEP 3
Execute the project with real-time communication, and deliver the final report promptly.
Related Services

 Fig.1 Illustration for Release-controlled curcumin proliposome. (Jia J, et al, 2016)
Fig.1 Illustration for Release-controlled curcumin proliposome. (Jia J, et al, 2016)
