The ability of lentiviral vectors to integrate stably in non-dividing and dividing cells, long-term expression of transgenes, and low immunogenicity make them ideal gene transfer vectors for gene therapy. CD Formulation provides lentiviral packaging services, which can help researchers to comprehensively solve the problems of viral outgrowth and low titer, targeting coding genes or non-coding genes to effectively increase the titer, with an average titer increase of more than 10-fold.
Technical Advantages of Lentiviral Packaging
- Wide range of infections. Lentivirus can effectively infect dividing and non-dividing cells and is suitable for almost all cell lines, such as neuronal cells, hepatocytes, cardiomyocytes, tumor cells, endothelial cells, stem cells, etc.
- A stable expression is possible. Lentivirus can integrate exogenous genes into the genome of host cells, which will not be lost with cell division, and can realize stable expression of target genes for a long time.
- High operational safety. Lentivirus adopts a self-deactivating replication-defective virus strain, which ensures operation safety.
Process for Lentiviral Packaging
Plasmid preparation
Prepare transfer plasmids, packaging plasmids, and envelope plasmids of adequate quality and purity.
Transfection of cells
Mix and add plasmids to cells using transfection reagents.
Culture and collection of virus
After transfection, cells need to continue to be cultured for a certain period of time (usually 48-72 hours) under suitable conditions. The culture supernatant containing virus particles is collected.
Virus removal and concentration
Remove cellular debris by filtering the viral supernatant through a 0.45 μm filter membrane. If desired, the virus can be concentrated using methods such as ultracentrifugation or polyethylene glycol (PEG) precipitation.
Virus titer assay
Determine the infectivity of the viral suspension using appropriate methods such as infection efficacy tests, fluorescent quantitative PCR, etc.
Virus storage
Dispense and store virus products at -80°C to maintain activity.
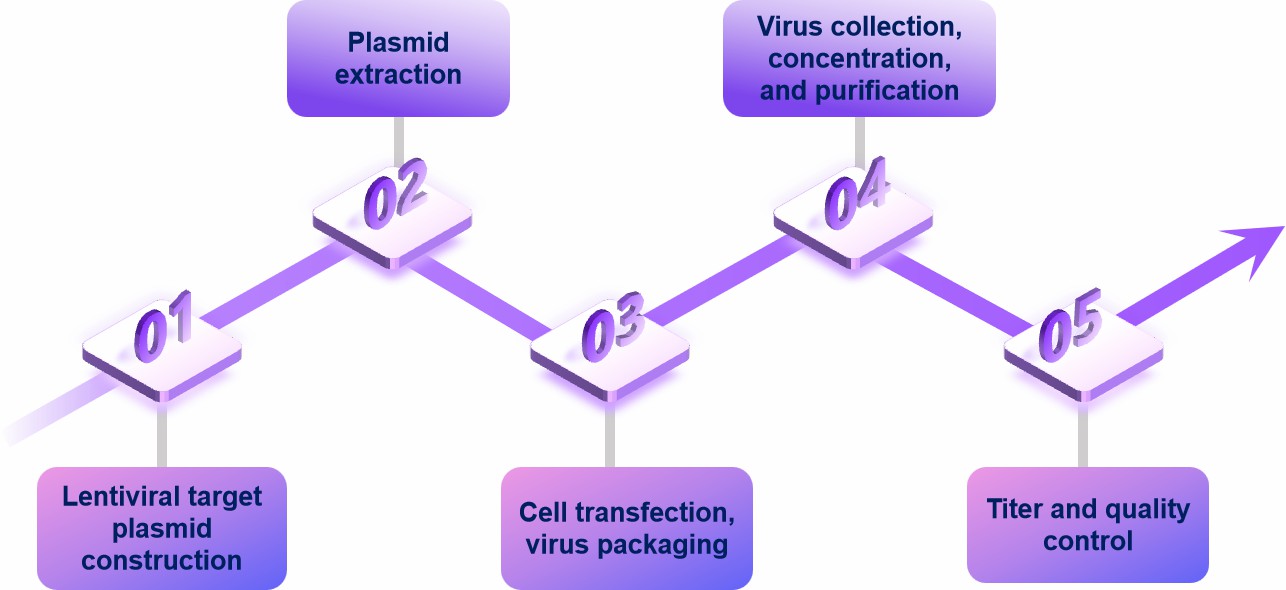 Fig.1 Lentiviral packaging process. (CD Formulation)
Fig.1 Lentiviral packaging process. (CD Formulation)
Optimizing Lentiviral Packaging for Gene Therapy
Viral packaging in lentiviruses has proven to be critical for the treatment of genetic diseases, cancer, and a range of other disorders. With rapid advances in biotechnology, lentiviral packaging technologies continue to be optimized and are expected to play an even greater role in the future of gene therapy.
- The constructed lentiviral vector is then subjected to a viral packaging step to produce viral particles that can infect cells and deliver the therapeutic gene. Viral packaging is usually accomplished in specially designed packaging cell lines that transiently express the proteins required for lentiviral packaging - such as Gag, Pol, and Env proteins. When the packaging cells are co-cultured with lentiviral vectors, they assemble these proteins into viral particles that are capable of infecting target cells and delivering genes.
- Concerning lentiviral packaging, a critical consideration is the production of lentiviral preparations with high titers (high concentrations of infectious viral particles) and high purity. This requires optimizing the conditions for virus production and purification, including optimizing transfection efficiency, increasing the yield of viral particles, and developing an effective virus purification protocol. Further, to minimize possible immune reactions and ensure patient safety, all non-essential viral proteins and impurities from the production process need to be removed.
- A major advantage of lentiviral vectors in gene therapy is their ability to efficiently transduce quiescent and dividing cells. This means that they can be used to treat a wide range of diseases, including blood disorders, metabolic disorders, neurodegenerative diseases, and muscle disorders. In addition, they tend to elicit a lower immune response than other types of viral vectors, which is particularly important for long-term treatment.
Our Platforms for Lentiviral Packaging
| Platforms & Technologies |
Content Description |
| Optimization of plasmid design |
We use self-inactivating (SIN) viral vectors, a design that reduces the risk of insertional mutagenesis and improves safety. And by adding enhancers and promoters to enhance the expression of target genes. |
| Cell line optimization |
We use highly efficient packaging cell lines that are optimized for efficient viral production capacity. Optimization of cell culture conditions can significantly increase lentiviral production by adjusting the culture medium, transfection reagents, and culture conditions. |
| Viral particle modification |
We enhance lentiviral targeting by increasing the type of envelope proteins of the viral particles and also use nanoparticles and other nanotechnologies to improve viral packaging and delivery. |
Highlights of Our Lentiviral Packaging
- We develop customized protocols to construct interfering lentiviral vectors and package viral particles for use in cell and animal experiments.
- The lentiviral-mediated genes we provide can be randomly integrated into the host cell genome to achieve efficient and sustained gene expression.
- According to the gene information and service requirements provided by customers, we can develop a program to meet customers' requirements and construct overexpression lentiviral vectors.
Published Data
Technology: Lentiviral packaging
Journal: Biotechniques
IF: 2.2
Published: 2020
This article describes a method to improve virus production and target gene knockdown using a modified pLKO.1 CMV pLKO.1C vector and optimized packaging construct ratios. Replacement of the pLKO.1 RSV promoter with a cytomegalovirus promoter produced titers that were on average three times higher than standard pLKO.1 packaged using a third-generation system, whereas optimization of the packaging vector ratios further improved titers, producing titers that were on average ten times higher than those of pLKO.1 packaged using a second-generation system.
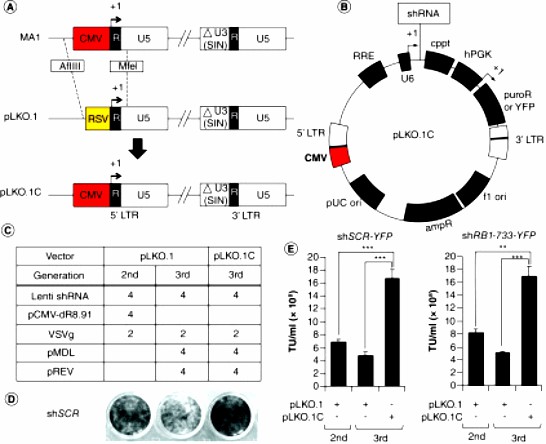 Fig.2 Improved transduction efficiency of third-generation lentiviral packaging. (Lee S, et al., 2020)
Fig.2 Improved transduction efficiency of third-generation lentiviral packaging. (Lee S, et al., 2020)
CD Formulation provides lentiviral packaging services that are carefully optimized to enhance biosafety, gene expression levels, and viral transduction efficiency. We have upgraded the lentiviral packaging process in terms of viral titer, purity, activity, and consistency. If you are interested in us, please feel free to contact us.
References
- Lee S, et al. Improved third-generation lentiviral packaging with pLKO.1C vectors. Biotechniques. 2020, 68(6):349-352.
Related Services

 Fig.1 Lentiviral packaging process. (CD Formulation)
Fig.1 Lentiviral packaging process. (CD Formulation) Fig.2 Improved transduction efficiency of third-generation lentiviral packaging. (Lee S, et al., 2020)
Fig.2 Improved transduction efficiency of third-generation lentiviral packaging. (Lee S, et al., 2020)